
Curious chemists first crossed paths with suberic acid back in the 1800s. They looked at it during their pursuit of understanding dicarboxylic acids from the breakdown of natural fats and the oxidation of various hydrocarbons. It didn't enjoy as much spotlight as some of its cousins, but its structure—eight carbons long with carboxyls on each end—raised interest among researchers exploring the chemistry of plant and animal tissues. By the late 19th century, suberic acid found a place as a tool for molecular structure studies and became a reference point in organic synthesis labs in Europe and America. Today, the world recognizes its CAS number (505-48-6), but its roots trace to old-school organic labs powered by glassware, trial and error, and genuine curiosity about how nature arranges carbon and hydrogen.
Suberic acid, or octanedioic acid, shows up as a white, odorless crystalline powder. On closer look, you’ll find a compound with the formula C8H14O4. Some labs get it in larger flakes, others in fine granules, but the essence stays the same. With a melting point around 141–143°C and a boiling point above 300°C, suberic acid asks for strong heat to budge. Its solubility feels like a middle ground: water takes it up slowly, alcohol and acetone make quicker work. In air, it doesn’t rush to react and holds up well against mild oxidation. The molecule stretches long enough to behave as a classic dicarboxylic acid but stops short of getting waxy or sticky.
Look at a technical sheet and you’ll see the dry numbers: purity hits upwards of 98%, moisture sits low, trace metals drop below detectable limits—important for pharmaceutical or specialty polymer work. Labels often include names like Octanedioic Acid, Suberic acid, and occasionally Suberinic acid. Some jars sit under synonyms such as 1,6-Hexanedicarboxylicacid or n-octanedioic acid, which can trip up even seasoned chemists if they don’t cross-check. Package sizes swing from neat glass bottles for the research crowd to bagged lots for the plasticizer trade. Detailed labeling standards help producers stay on the right side of import/export rules and keep everyone safe in the process.
Factories lean on two main methods: oxidation of octanol or hydrogenation of suberic anhydride. For the serious industrial crowd, the oxidation route starts from cyclooctanol or cyclooctanone, using nitric acid and a bit of heat. Yields can reach 70–80% if you dial in temperature and pressure just right. Smaller-scale chemists often choose oxidative cleavage of sebacic acid or extract it from certain alkaloids. The process leans into established organic chemistry—cautious handling of strong acids and controlled atmospheres to keep everything predictable. Over repeated runs, subtle tweaks in catalyst concentration or agitation speed can mean the difference between clean acid and a mess of colored byproducts.
Put suberic acid on the bench and chemists see opportunity. Both ends present carboxyls ripe for turning into esters, amides, or anhydrides. Esterification with various alcohols yields flexible plasticizers or flavor intermediates. Lab teams make polyesters out of it, using it as a linking unit in chains that add resilience to materials—crucial in specialty plastics and biodegradable films. Drop lithium aluminum hydride on it, and suberic acid gets reduced to octanediol, opening another alley for applications. Add formaldehyde and get condensation products useful for advanced polymer work. Every slight chemical tweak rewrites what suberic acid can become down the line.
Chemists have a habit of naming things in layers. Suberic acid pops up in literature as Octanedioic Acid, n-Octanedioic acid, Suberinic Acid, and 1,6-Hexanedioic Acid. Rarely, some catalogs list it as Diprotic octa-acid. These aliases show its spread across countries and industries. Some old pharmacy recipes from the 20th century even called it “Horseradish acid” due to early plant extraction work. Keeping track of these names matters whether reading vintage papers or sourcing modern samples since subtle differences in nomenclature can steer research or production the right—or wrong—way.
Every bag or bottle ought to arrive with guidance: suberic acid doesn’t explode or burn easily, but routine lab care still rules the day. Its dust irritates skin and eyes after repeated contact. Swallowing even small amounts isn’t a good idea. MSDS sheets stress gloves and goggles, plus solid ventilation where powders could get airborne. For folks in production plants, dust control keeps air safe and storage in tight containers prevents moisture uptake. So far, it doesn’t show acute environmental harm, but manufacturers still set up containment and run monitoring to prevent floor spills from reaching drains. I remember handling it in graduate school, and even though I never saw it cause a reaction, standing practice had everyone respect its potential just as much as its proven properties.
Polymers, resins, and plasticizers count on suberic acid for strength and flexibility. Chemists in the synthetic fragrance world use it as an intermediate, building up scents from simpler chains. Some pharmaceutical researchers use its derivatives to create controlled-release capsules. Agrochemical companies slide it into specialty plant protectants, aiming for slow, controlled breakdown in soil. Suberic acid also lands in electrochemistry as an additive to improve electrolyte stability. Over the past decade, I watched the push for biodegradable plastics spark fresh attention, with suberic acid entering blends designed for compostability without giving up durability. Each application calls for a careful balance of cost, purity, and reactivity—no one-size-fits-all solution, just a range of needs that this eight-carbon acid can meet.
Academic and industrial labs don’t treat suberic acid as just another background chemical. Researchers track its performance in specialty polyesters, giving designers of high-end composites new tools to work with. Biomedical studies focus on using its structure to tune drug delivery systems, especially in hydrogels and microcapsules. Surface chemists experiment with tweaking its chain to bind better to metal oxides, searching for better corrosion inhibitors. Last year in our collaborative project, we spun up several suberic acid-based co-polymers for environmental sensors—each tweak nudged sensor life or sensitivity a little higher. Journal output on suberic acid shows steady growth, pointing to its ability to evolve with new technology and changing industry requirements.
Suberic acid rates as relatively low-risk at typical exposure levels. Animal studies peg its oral LD50 higher than many solvents but lower than benign table salt. Chronic studies show mild organ effects only at deliberately high dosages. In cell studies, the acid form doesn’t easily cross membranes or trigger strong inflammatory responses. Disposal guidelines encourage careful transfer to waste containers and neutralization in case of liquid spills. European and American regulators don’t list it as a persistent pollutant or known carcinogen, which makes its use attractive in applications where human and environmental safety get close scrutiny. Still, good practice never stops evolving, and ongoing toxicology work could yet reveal sensitivities—reaction products from its industrial processing, for example, deserve a thorough look before scaling up production.
Interest in renewables and greener plastics gives suberic acid a shot at wider adoption. Biotechnologists eye routes for fermentative production, aiming to sidestep petrochemicals altogether—a move that could bring its carbon footprint down and make biodegradable plastics more cost-competitive. If pilot projects can push yields from renewable feedstocks high enough, supply chains for everyday products could shift in a direction friendlier to our planet. In materials science, the push for tailor-made polyesters gives suberic acid another lease on life, since its backbone length fits the sweet spot between flexibility and toughness. Academic research pushes boundaries in self-healing materials, where modified suberic acid units help blend mechanical robustness with chemical adaptability. Investments in these directions keep rising—so long as new findings deliver better properties or provide a pathway to safer, more planet-friendly products, suberic acid stands ready for another century of innovation.
Suberic acid, also known as octanedioic acid, brings together two carboxyl groups on an eight-carbon backbone. In plain terms, it’s a dicarboxylic acid with a chain long enough to bridge gaps in many different products. If you work with synthetic materials, you’ve probably run across it in a lab or perhaps read about it as one of the “building blocks” for more complex molecules. The power of suberic acid, for me, sits in that flexible backbone and how it helps engineers and chemists solve everyday challenges.
Nearly every time chemical engineers set out to design a plastic with specific flexibility or strength, they turn toward dicarboxylic acids like suberic acid. Its unique combination of length and reactivity lets it form tough yet adaptable bonds with other monomers. If you’ve handled soft plastics or synthetic leathers, you’ve probably benefited from suberic acid, even if its name never reached the packaging.
Nylon polymers—strong, lightweight, and used everywhere from toothbrushes to high-end car parts—draw from suberic acid’s chemical features. Companies favor certain dicarboxylic acids over others, depending on how strong, bendable, or water-resistant they want their final product. Suberic acid may not headline the ingredient list, but its impact can’t be ignored in these blends.
Medicine seems far removed from plastics, yet suberic acid plays a part in advancing drug delivery. Drug researchers sometimes use special suberic acid-based compounds to attach drug molecules to carriers that linger longer in the body, or release slower over time. These tweaks lead to fewer doses for patients, steadier levels of medication, and less frequent unpleasant side effects.
Suberic acid also acts as a linker in certain diagnostic tests and treatments. It can anchor molecules to surfaces—such as medical chips or test strips—so doctors get reliable results. These details may sound small but they build the backbone of dependable devices and better patient experiences.
Many folks forget about the acids inside laundry detergents, shampoos, and conditioners. Suberic acid helps stabilize these mixtures, making them smooth and easy to rinse. The long chain in its structure provides both strength and softness. In my home, everyday products with more gentle cleaning power often include derivatives of these dicarboxylic acids, helping remove residue without wrecking fabrics or skin.
Cosmetics manufacturers, always seeking that silky texture and safe, skin-friendly formula, tap into suberic acid and similar compounds. These acids appear in lotions and creams where both durability and absorption mean happy customers.
Sustainability has become the talk of every table. As concerns over fossil fuel–based products grow, so do efforts to create suberic acid from renewable sources. Some startup companies experiment with bio-based fermentation processes using plant sugars and specific microbes. This approach aims to drop the carbon footprint while keeping costs in check. Success in these new production methods could shift not just how plastics are made, but even the packaging in our homes and the fibers in the clothes we wear.
For those looking for simple improvements, switching to materials shaped by substances like suberic acid brings both performance and a path to greener options. That’s something everyone can get behind, whether building cars, making medicines, or just tackling the next load of laundry.
Suberic acid lands in the middle ground of chemical handling. Working in the lab, I ran across it more often than the big-ticket acids like sulfuric or hydrochloric, but rarely did it get much attention. You find suberic acid (octanedioic acid) in research on nylon and plastics, and sometimes sprinkled through textbooks on biochemistry, simply because it's part of the dicarboxylic acid family.
On paper, suberic acid has a low toxicity profile. It’s not acutely toxic or likely to cause severe immediate harm if it gets on skin. That doesn’t mean it belongs next to baking flour, though. It has an abrasive, gritty texture, and if you’re not careful, it dries the skin and might cause mild irritation or rash after repeated contact. Inhaling the dust can irritate lungs. During college, I watched more than one classmate turn their nose up after a cloud of poorly handled powder got loose in an open room.
A lot of safety depends on how you’re using it. If you’re dumping kilos of suberic acid into industrial machinery, you want gloves, eye protection, maybe a lab coat and dust mask to steer clear of airborne particles. The same goes if you’re in a tight workspace where spills don’t get noticed until your hands start tingling.
Take the time to read the Safety Data Sheet. Every container I’ve opened had basic handling instructions printed right on the label. Simple measures—gloves, goggles, avoid breathing in dust—have kept me safe every time. I found chemical burns from suberic acid rare in the extreme. It’s nothing like sulfuric acid, which chews through flesh within seconds.
Nobody likes the idea of sneaky long-term risks. Thankfully, suberic acid sits near the bottom of EPA watch lists. There’s no evidence pointing toward cancer, mutations, or reproductive hazards. That’s a big relief for folks using it often. At the same time, I’ve noticed that skin drying can get worse with repeated daily exposure. Keeping up that barrier—washing off after use, and wearing gloves—goes a long way. One coworker preferred latex gloves, but I had better luck with nitrile, which feels more reliable on thin, dry acids.
Don’t let the benign label trick you into carelessness. It’s worth keeping suberic acid jars closed tight and away from moisture. In my grad school lab, I once found an open jar caked with crystals, sticking to shelves and attracting mess. That attracts trouble. Spilled powder is easy to sweep up, but it gets everywhere fast, and no one likes surprising powder clouds.
If you’re not sure, treat suberic acid with respect. Standard chemical hygiene—protective equipment, good ventilation, clear labeling—works like a charm here. It doesn’t cause instant emergencies, but it deserves attention. Skin irritation and mess make for extra cleaning, and a little care prevents that. Treat it as you’d handle any mildly irritating chemical: gloves, clean workspace, and an eye on the warning label.
Nothing beats training and a few common-sense precautions. Talk to coworkers, keep safety instructions visible, and report spills as they happen. In decades of collective experience, that approach kept the problem cases to a bare minimum. The science lines up with everyday know-how—smart handling keeps you out of trouble.
Walk into a chemistry lab, and you’ll see plenty of curious names. Suberic acid sounds like one of those obscure, lab-coat only substances. But suberic acid, known by its formula C8H14O4, connects much more to industry and daily life than most folks realize.
Take any bottle of suberic acid and what’s inside breaks down to eight carbon atoms, fourteen hydrogen atoms, and four oxygen atoms. This simple makeup gives suberic acid unique traits. In fact, it’s a dicarboxylic acid, which means it carries two carboxyl (–COOH) groups. Having these two reactive ends brings extra chemical versatility.
Suberic acid naturally appears in trace amounts in some plants and roots, but people mainly know it from the lab or manufacturing setting. The wider world views it as a building block for more complex molecules. Its chain of eight carbon atoms sets it apart from shorter cousins like succinic or adipic acid, giving it a place in certain plastic and polymer applications.
Plenty of nylon types, polyamides, and plasticizers trace their origins to acids like suberic acid. Manufacturers depend on it for creating resilient, flexible materials. Its presence means better resistance to moisture, chemicals, and regular wear.
Companies prize suberic acid for making textiles smoother and longer-lasting. Adhesives, perfumes, and lubricants often use it as a stabilizer or an intermediate. Road salt sometimes contains derivatives. Small amounts even shape certain medical creams, thanks to its ability to adjust a product’s pH or act as a mild preservative.
Making pure suberic acid means exacting processes. Chemists often run oxidation reactions using castor oil or similar raw materials. Each batch demands careful control to keep byproducts away. Suberic acid doesn’t bring severe safety hazards; handled with basic gloves and goggles, it behaves predictably. But dust or high concentrations may irritate skin or lungs.
On a personal note, my own stint in college labs taught me respect for even “simple” substances. One distracted moment with an acid solution and you learn quickly about proper handling and labeling. Labs worldwide follow clear protocols with acids like suberic to keep incidents rare.
Sustainability draws more industry attention every year. Since suberic acid starts with petrochemical or plant-based sources, companies eye newer, greener ways to produce it. Processes that use less energy or generate fewer toxic leftovers sit high on producer wish lists.
At the same time, people should care about where their products come from and what chemicals ride along in those shoes, textiles, or even pharmaceuticals. Regulatory bodies have set safe limits and guide proper disposal. Efforts to create biobased suberic acid using engineered microbes hint at a future with fewer emissions and lower waste.
Some promising steps involve enzymes as catalysts to replace harsh conditions. Researchers aim for upcycled plastic waste to yield raw materials like suberic acid. Transparency, strong safety culture, and tighter controls at every stage will drive better outcomes. Companies and communities can share data and best practices for smaller environmental footprints.
Staying informed and supporting responsible manufacturers makes a difference. Watch for third-party certifications, read labels, and push for continuous innovation in specialty chemicals like suberic acid.
Suberic acid pops up in some uncommon corners of chemical manufacturing. A white, crystalline powder, it carries its own risks. No one wants to deal with an accidental spill because someone tossed a bag on a damp shelf. This material won’t bite hard like some brutal acids, but it has its quirks. Direct skin contact can lead to irritation, and improper storage can ruin batches, spark compliance headaches, or even trigger safety violations.
I’ve seen labs waste valuable time and money cleaning up avoidable messes because guidelines got ignored. A misplaced jar can lead to product contamination or unstable conditions. For any lab manager or technician, being careless with acids—even the less volatile ones—can turn a workday upside down. Years spent in lab environments taught me the value of simple routines and clear, enforced labeling practices. Sometimes, the difference between a smooth operation and a mess lies in a simple, overlooked detail like where the container sits.
Use airtight containers made of materials that won’t react with the acid, such as glass, polytetrafluoroethylene (PTFE), or high-density polyethylene (HDPE). Favor dry spots over places that trap humidity. Exposure to moisture leads to clumping and spoilage, ruining the powder’s usefulness and potentially harming equipment during later processing. Keep it away from direct sunlight; suberic acid stays stable best at consistent, cool room temperatures. In one old lab, a researcher left a bottle by a sunlit window all summer, only to return and find the contents fused together. Not a good, or safe, way to learn a lesson.
Ventilation matters. Suberic acid won’t off-gas like some volatile chemicals, but fine particles can get airborne during transfer. Use low-traffic shelves, preferably at eye-level, to reduce the chance of spillage or contamination. Avoid high humidity zones—never next to water baths, steam lines, or beneath leaky ceilings. I recall a humid week pulling in extra fans because the air was thick and absolute chaos for powdered reagents. Just one forgotten jar turned into a sticky, semi-liquid mess nobody wanted to clean.
Clear labeling protects staff. A decent label should list the full chemical name, concentration (if mixed), hazard details, and the date received or opened. I’ve watched new hires confuse containers without good labels, leading to time-consuming audits and thrown-out supplies. Adding a line on proper handling gives relief for the next shift, boosting compliance almost overnight. Every person grabbing a container should know its story at a glance—no guessing games.
Track all movements in a simple log or digital system. Missing acids create confusion and can put compliance status at risk during inspections. Chemical storage only works when every item has a reliable paper trail. Years ago, lost inventory threw off entire project timelines, mostly because someone borrowed a jar without signing it out. That kind of mistake costs real money.
Make dry storage a shared policy, not just a personal habit. Use dehumidifiers or silica gel packs in risky climates. Train staff to spot leaks or condensation near storage shelves. If a container cracks or warps, replace it right away. Rotate stock regularly and check for visible changes like caking or color shifts.
Invest in storage that matches both the scale and risk. Even if suberic acid isn’t the most dangerous chemical on the shelf, strict storage habits keep everything—and everyone—a lot safer. Consistent routines save money, protect equipment, and make inspections less stressful. Simple, focused attention beats troubleshooting emergency spills every time.
Suberic acid is not something found in the corner drugstore or the big-box grocery. Its name pops up in chemistry labs, research projects, and some manufacturing settings. Students probably won’t run across it in basic science classes, but folks in pharmaceuticals or advanced materials might use it for specialized processes, mainly because of its unique properties as a dicarboxylic acid.
The straightforward way to get suberic acid is through chemical suppliers. There’s no need to hunt around in local stores; companies like Sigma-Aldrich, Alfa Aesar, Thermo Fisher Scientific, and TCI carry it. These suppliers expect you to provide business credentials, such as a laboratory address or proof you represent an institution, since chemicals like suberic acid don’t end up in household products for a reason.
If you search online, Jam-packed marketplaces like Amazon or eBay sometimes offer listings, but caution is key since chemical sourcing through unofficial channels risks quality issues, wrong labeling, or noncompliance with laws. In my own experience, for any project that touches research or medical development, sticking with established suppliers is the way to keep standards intact and avoid safety headaches.
Suberic acid’s handling isn’t as breezy as buying baking soda. Many substances used in lab environments are regulated because of health risks and the technical knowledge needed to use them properly. Reputable suppliers require documentation: proof you know what you’re doing and you’ll use these chemicals safely. Years ago, in a research group I worked with, receiving even basic chemicals demanded paperwork, and the safety training that came with it helped prevent accidents.
Some regulations are strict for a reason. Suberic acid isn’t wildly dangerous in small quantities, but powdered organic acids must get the right treatment, with correct labeling, storage, and disposal. Clear rules help everybody avoid mishaps. Responsible companies help customers comply with regulations, making the buying process more reliable.
Most people asking about suberic acid aren’t hobbyists—they’re researchers, educators, or industry professionals. This substance appears in biodegradable polymer research, pharmaceutical intermediate synthesis, and studies exploring new types of coatings. The target audience is small, but outcomes can shape products most folks use every day, from safer plastics to better medications.
Research groups need to know exactly what’s in each bottle, expecting high purity and traceability. Anything less can wreck months of effort or introduce hazards. Over the years, I’ve seen how a batch with unknown impurities can wipe out the trust in results, so reliable sourcing isn’t just a formality—it’s mission-critical.
People sometimes feel frustrated with paperwork, price tags, and restrictions tied to small-quantity chemical sales. Streamlined online vetting systems help researchers buy what they need without long delays. Easy-to-use safety guides and transparent regulatory steps on supplier websites also build trust.
Community networks play a role, too. Local universities sometimes pool orders, letting smaller labs get materials responsibly. These collaborations reduce costs and open doors for people outside major institutions. That said, the checks and balances built into the system keep quality and safety from slipping. If you’re just starting out or feel overwhelmed, linking up with a local lab or research network gives clarity and helps navigate both purchasing and safety questions.
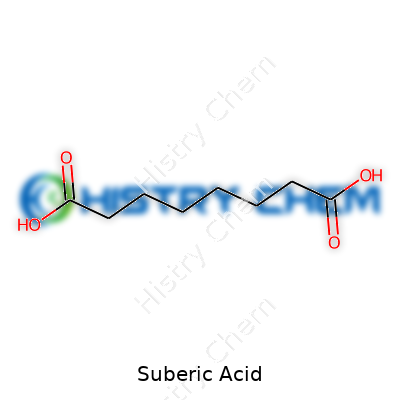
| Names | |
| Preferred IUPAC name | octanedioic acid |
| Other names |
Octanedioic acid
Suberic acid 1,6-Hexanedicarboxylic acid |
| Pronunciation | /ˈsuːbərɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 505-48-6 |
| Beilstein Reference | 778367 |
| ChEBI | CHEBI:15711 |
| ChEMBL | CHEMBL1436 |
| ChemSpider | 5363 |
| DrugBank | DB04262 |
| ECHA InfoCard | 088b4055-ff63-3a8b-9099-49e91ca702d9 |
| EC Number | 204-673-3 |
| Gmelin Reference | 72368 |
| KEGG | C02445 |
| MeSH | D013370 |
| PubChem CID | 8682 |
| RTECS number | WH6650000 |
| UNII | WI4X0X7BPJ |
| UN number | UN3265 |
| Properties | |
| Chemical formula | C8H14O4 |
| Molar mass | 146.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.28 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -0.838 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.41 |
| Basicity (pKb) | 1.92 |
| Magnetic susceptibility (χ) | -6.28×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.437 |
| Viscosity | Viscosity: 3.12 mPa·s (at 150 °C) |
| Dipole moment | 1.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 204.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1441.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4086.7 kJ/mol |
| Pharmacology | |
| ATC code | A16AB16 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS07; Warning; H315, H319, H335 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | 181°C |
| Autoignition temperature | 600°C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral Rat 7400 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 4,280 mg/kg |
| NIOSH | NA0525000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 300 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Azelaic acid
Pimelic acid Sebacic acid Adipic acid Glutaric acid Succinic acid |