
Chemists working with dicarboxylic acids began to seek new derivatives during the growth of polymer science in the twentieth century. Sebacic acid, a fairly common ingredient in the nylon and plastic world, found new life as scientists hunted for compounds that offered more than just flexibility or heat resistance. Sebacic dihydrazide slipped into the spotlight through the tireless work of those studying hydrazide-based additives for polymers, coatings, and specialty chemicals. Manufacturers once focused solely on volume polyamides now saw the value in niche components. Sebacic dihydrazide emerged from that laboratory scramble. Its value was clear to anybody dealing with customizable materials in paints and adhesives—there was constant demand for safer and more stable curing agents. The learning curve behind this compound drew on decades of mistakes and successes with hydrazines and sebacates before anyone started using it on a meaningful scale.
Sebacic dihydrazide comes as a solid, typically odorless and white. Its main draw centers on its strong crosslinking ability as a curing agent, especially in waterborne epoxy systems. Producers often pack it in fiber drums or heavy-duty bags to keep dust down and reduce exposure, as workers tighten up handling procedures. Sales mostly target the specialty resin industry, laboratories, and those needing stable curing agents that don’t set off dangerous side reactions. Compared to standard hardeners, sebacic dihydrazide can give paints, potting compounds, and polymer coatings a unique balance between weather resistance and manageable cure schedules. Most material safety data sheets point out its main uses and the need to respect dust limits during processing or mixing.
At room temperature, sebacic dihydrazide remains a stable white powder, dissolving poorly in cold water but moderately in hot water and some polar solvents. It melts above 185°C, so high-temperature conditions rarely cause problems unless neglect creeps in during warehouse storage. The compound stays fairly inert in properly sealed containers and won’t break down from light or mild humidity. Its hydrazide groups react predictably with epoxies and aldehydes, making it less of a headache than more reactive cousins in the hydrazine family. Reactivity levels suit most industrial and research-grade requirements—no runaway reactions, no bizarre byproducts under normal manufacturing scenarios.
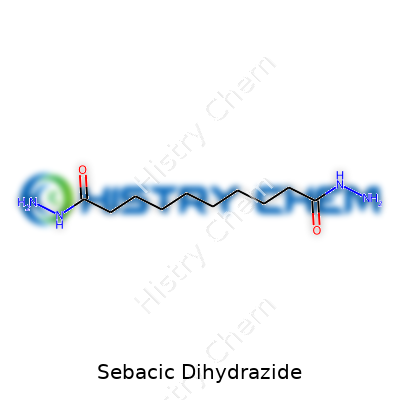
Labels for sebacic dihydrazide must display its systematic name and chemical formula: C10H22N4O2. Good manufacturers include batch purity, usually tested by high-performance liquid chromatography or melting point range. Color description remains important—yellow tint suggests problems with stability or impurities. Labels often list permissible dust exposure limits and basic safety warnings, pushed by tightening regulations in chemical handling. Sellers and importers carry extra obligations for hazard communication, especially for labs or purchasers new to hydrazide curing agents. The technical sheet tells customers what testing protocols to follow and flags incompatible substances, usually strong oxidizers or acids. Precision in labeling allows processors to sidestep unwanted contamination or mix-ups during product changeovers.
Companies usually make sebacic dihydrazide by treating sebacic acid or its esters with hydrazine hydrate. The process uses moderate heat and careful addition sequences to control exothermic reactions and avoid flooding the reactor with volatile gases. High-purity water washes, followed by filtration and drying under controlled temperatures, fix most problems with residual solvents or unreacted materials. Some big plants automate the filtration step. Key to the process: using fresh, high-purity hydrazine, since contaminated feedstock leads to unstable batches. Process engineers track temperatures and agitation speeds, shifting them to maximize product quality without spiking costs or hazards. Experience makes the difference here—minor tweaks in raw material can change end-product shelf life or reactivity.
Sebacic dihydrazide’s defining feature is the pair of terminal hydrazide groups. These groups grant it a talent for reacting with epoxides, aldehydes, and a handful of activated esters. Most industrial chemists appreciate that it won’t start unwanted side reactions with most standard fillers, pigments, or anti-corrosion additives. For custom work, substitutions at the alkyl backbone or N-alkylations can alter flexibility, cure speed, or even resistance to water and solvents. Its reactivity with epoxy resins drives its main commercial use—custom transports and high-build coatings—since it crosslinks rapidly at moderate temperatures. Specialty research sometimes pushes the molecule further, working on conjugations that attach bioactive ligands or magnetically responsive tags, but most commercial efforts stick with the reliable base molecule.
Walk through a warehouse or scan a supplier’s catalog, and you’ll glimpse names ranging from sebazyde to sebacohydrazide and N,N’-hydrazinediylbis(nonandiamide). Some companies stick with the CAS number (108-94-1) for reordering and clarity. Old industry catalogs also call it sebacoyl dihydrazide or just SDH, especially in Asian and European distribution circles. The range of synonyms caused confusion with regulators before standardized labeling set in a few years back. Hydrazide chemistry, in general, spawns a forest of similar-sounding names, so most labs clarify with chemical structure on internal stock cards to avert errors.
Sebacic dihydrazide’s low volatility lowers the odds of occupational exposure, but chronic inhalation of powder or casual ingestion still introduces real risks. Hydrolytic breakdown in the gut can release free hydrazine, which worries industrial hygienists. Plants running hydrazide operations maintain strict dust control policies, with local exhaust ventilation and personal protective equipment as the backbone of workplace safety. Staff undergo regular training, especially workers mixing or heating the product. Recent guidance from chemical safety authorities urges real-time dust monitoring systems, monthly lung function checks for high-exposure roles, and secure, labeled containers at all transfer points. In-level labs and contract manufacturers often build spill kits and fire suppression into SOPs. Safe disposal depends on incineration under controlled conditions—landfill dumping brings liabilities nobody wants. Emergency procedures focus on prompt washout and medical attention for any direct skin or eye contact.
Sebacic dihydrazide solves problems for people in paints, adhesives, and specialty plastic industries. Paint formulators see predictable cure times and improved weather resistance without noxious odors or fast exotherms. Adhesive manufacturers use it to bridge gaps in metals, ceramics, and plastics—the hydrazide backbone supports flexible yet strong bonds, especially where thermal cycling threatens weaker linkers. Advanced materials labs use it in solid polymer electrolytes and membrane materials, aiming at new energy storage or filtration tech. A growing trend features sebacic dihydrazide in eco-friendly coating systems, responding to regulations on volatile curing agents and environmental toxins. Waterborne epoxy coatings, a niche but fast-expanding field, lean heavily on its attributes. At research conferences, polymer chemists discuss its role in self-healing materials or advanced drug release platforms, where predictability in degradation profiles matters more than simple strength.
Academic chemists and industrial labs keep searching out better methods for producing and modifying sebacic dihydrazide. Lowering costs while improving yield pushes much of today’s research. Some teams look for ways to streamline purification—membrane separations or advanced solvent-free synthesis hold promise. In application labs, testing continues on novel compositions: blending with biopolymers, pairing with functionalized nanofillers, and building in plasticizers without sacrificing the product’s reactivity. There’s a move toward green chemistry, nudging manufacturers to swap out hazardous solvents for safer alternatives. Material scientists, looking for improved heat and hydrolysis resistance, keep experimenting by tweaking side chains or combining sebacic dihydrazide with co-curing agents. Intellectual property filings show patents climbing, especially in high-end paints and specialty engineering plastics.
Since the hydrazide functional group traces its reputation back to potent toxins like hydrazine itself, toxicologists do not sleep when new uses emerge. Animal studies on sebacic dihydrazide show relatively mild acute toxicity, mainly at high doses or through direct injection. Chronic inhalation studies confirm that the most common hazards involve the respiratory tract and, for longer exposures, the kidneys. Regulatory agencies watch data coming from occupational studies, pushing for routine reassessment. Dust inhalation limits, even with modern ventilation, get revised downward if new findings suggest tissue accumulation or slow breakdown. So far, epidemiological surveys in major plants haven’t flagged unexpected cancer links or reproductive hazards. Because hydrazine compounds sometimes act as mutagens, researchers look at the molecule’s structure after digestion or hydrolysis to confirm breakdown products stay well away from dangerous thresholds. Biomonitoring studies for plant workers support regular updates to permissible exposure limits and shape the next round of safety briefings.
Markets for sebacic dihydrazide reflect trends in safer, lower-toxicity curing agents and growing demand for waterborne epoxy coatings. Regulations on toxic curing materials and environmental sustainability drive greater research investments. Smart coatings and functional polymers for automotive, construction, and electronics sectors guarantee continuing relevance. Demand for more eco-aware hardeners intersects with chemists’ push for lower-energy synthesis and recycling—so innovations in closed-loop processing catch industry interest. Beyond coatings and adhesives, niche applications trickle into medical device coatings and battery technology, due to the molecule’s stability and customizable backbone. As cleaner technologies emerge, companies look for ways to reuse or recycle materials instead of defaulting to incineration. Funding for R&D in advanced materials and sustainable chemistry will only increase. Sebacic dihydrazide stands as a reference point for how small chemical tweaks can deliver real improvements in the safety, stability, and environmental impact of many familiar materials.
Not everyone walks into a hardware store or picks up a new phone case and thinks about the chemistry involved. Yet, tucked into formulas behind the scenes sits sebacic dihydrazide, a chemical that plays a bigger role than most folks realize. Factories across the world bring it in for good reason: it helps give coatings and plastics qualities that truly matter to users.
Think about glossy tables or tough, chip-resistant paints. Manufacturers aim to build surfaces ready to stand up to heat, water, or accidental scrapes. Sebacic dihydrazide lets them build those layers. This compound, derived from a long-chain dicarboxylic acid, often gets added to powder coatings because it crosslinks with polymer resins at relatively low temperatures. The outcome? Surfaces that handle a lot more abuse compared to old-fashioned paint jobs.
People worry about the air quality inside their homes and offices, so companies are searching for ways to reduce solvents in paints and coatings. Sebacic dihydrazide allows for those powder-based coatings, cutting down on smelly chemicals and offering environmentally safer results. Data from the European Coatings Journal has flagged a clear trend toward powder coatings over traditional solvent-based options, partly fueled by compounds like sebacic dihydrazide.
It’s easy to overlook plastics and adhesives in our daily routines—until they fail. Sebacic dihydrazide shows up in certain glue formulas, where it helps with setting and improving resistance to moisture. No one enjoys the sight of a peeling kitchen countertop or a leaky seal in the bathroom. This compound brings stability and flexibility without making adhesives tricky to use.
Medical products also see benefits, particularly in controlled drug release systems. Because sebacic dihydrazide is linked to biocompatible materials, researchers use it for devices that slowly release medicine over time. Medical journals have reported on its value for hydrogels and films, giving doctors safer options for tough cases like chronic wounds.
No chemical solution feels complete unless people check on toxicity and how substances behave both in the factory and outside it. The industry’s track record for sebacic dihydrazide rates as relatively safe for people to handle, according to chemical safety boards. Yet, the onus rests on companies to observe strict guidelines and keep manufacturing responsible.
Sustainability crops up as a real-world concern. Sebacic acid, the base for this compound, comes from castor oil. Castor beans grow in several parts of the world with fewer inputs compared to fossil-based chemical feedstocks. That gives sebacic dihydrazide an edge for folks watching enviro footprints.
Folks working in coatings or plastics can’t ignore customer demands for safer, tougher, longer-lasting products. Sebacic dihydrazide helps meet these calls, but companies should continue to invest in research. Developing more bio-based pathways, tightening safety controls, and being transparent about chemical sourcing may keep this compound in good standing as new regulations take hold. The mix of performance, relatively safe handling, and its plant-based origins puts sebacic dihydrazide on the radar for any company aiming to innovate responsibly.
Sebacic dihydrazide isn’t something you find on a grocery shelf, but it stands out in the chemical world. Built from the union of sebacic acid and hydrazine, the resulting white, powdery compound feels smooth to the touch and has a mild, almost undetectable scent. Most people outside laboratories never cross paths with it, but plenty of industries depend on its unique performance for their products.
Each molecule comes packed with two hydrazide groups at either end of a ten-carbon chain. The structure makes sebacic dihydrazide fairly stable, even under changes in temperature or exposure to air. In practice, its melting point sits comfortably above 180°C, standing up to heat during manufacturing steps where weaker substances would break down. This heat resistance keeps it intact in all sorts of applications, from specialty resins to powder coatings.
It dissolves easily in water—no elaborate lab tricks needed. That trait feels small until you think about how many reactions work only if a chemical can mingle evenly in water. This water-soluble nature makes sebacic dihydrazide a reliable choice for aqueous processing, helping it blend into paints, adhesives, and other water-based mixtures.
This chemical doesn’t just sit around. It reacts with carbonyl-containing compounds like aldehydes and ketones, forming hydrazones. This reaction seems technical, but the practical upshot lies in how it lets scientists crosslink polymers or detect carbonyls in analytical labs. In coatings and inks, forming these bonds gives toughness and weather resistance. The backbone of sebacic dihydrazide helps create tight, durable polymer networks.
Sebacic dihydrazide’s reputation for low toxicity and mild odor makes it easier to handle than many alternatives, especially in settings where people worry about fumes or residue. Still, it contains hydrazide groups, and those can cause irritation or allergic reactions in some people. As with any fine chemical, gloves and a mask offer smart protection off the bat. MSDS data sheets recommend keeping it away from food and skin, just for peace of mind.
Manufacturers use sebacic dihydrazide because it brings several key properties without creating tricky byproducts. Polyurethane chemists lean on it as a curing agent, helping flexible coatings and foams hold up to stress. Ink and adhesive industries use it for its water compatibility, low odor, and fine grain size, improving the texture and finish of products. Some polymer labs put it to work creating custom crosslinking density in resins, dialing in flexibility or hardness. It has even shown up as a stabilizer, lengthening the shelf-life of sensitive chemical mixtures that might otherwise degrade in storage.
Industrial use always throws up challenges. Spill management and air monitoring cut down on accidental exposure. Researchers continue looking for greener production pathways, minimizing energy and waste in manufacturing. Careful recycling and disposal prevent environmental build-up. In development labs, folks push for spotting and eliminating even lower-level residues, ensuring downstream products stay clean and safe for every end user.
Too many chemicals work in the background, shaping everyday reality without much attention. Sebacic dihydrazide, by blending structural strength with water solubility and mild handling risk, delivers consistent value across different sectors. Continued monitoring, better manufacturing controls, and straightforward safety steps keep its future secure in the chemical toolbox.
Sebacic dihydrazide, a white and odorless powder, pops up in places that may surprise most of us. Manufacturers put it into epoxy and polyurethane resins, aiming to create coatings that stand up to wear and tear. I remember first seeing its name on a list of crosslinkers while exploring options for non-toxic adhesives. The chemical world is full of tongue-twisters, but its presence in our daily environment calls for a closer look. So, is it dangerous to our health or to the planet?
Research into sebacic dihydrazide safety isn’t as robust as for some legacy industrial chemicals. Most databases, like the ECHA and the United States EPA, recognize it and have records for it in industrial use. Companies and regulatory agencies run tests that focus on eye and skin irritation, inhalation toxicity, and long-term effects.
Reports show that sebacic dihydrazide has low acute toxicity. Swallowing small amounts or having it on the skin by accident doesn’t usually lead to immediate danger in lab animals. It can slightly irritate the eyes or skin upon direct, repeated contact, but not at levels that raise major red flags. The big difference here compared to some widely used curing agents or accelerators is that it lacks severe red-label warnings or global concern lists attached to it.
Scientists often point out the absence of robust, peer-reviewed research on long-term human exposure to sebacic dihydrazide. It’s one thing to pass standard short-term tests. Long-term, low-dose risks take years to show up, both in people and in the environment.
Sometimes people focus solely on acute toxicity and miss subtle risks. For instance, repeated handling of powders in under-ventilated workplaces can lead to unexpected respiratory symptoms, even if safety sheets say “low toxicity.” Protective gear isn’t just for show. Factory workers told me stories where fine powders, rated "non-toxic," aggravated asthma or dermatitis over weeks or months. Health risks often slip through because each study looks at a narrow piece of the puzzle.
Sebacic dihydrazide looks better than many older chemicals—especially metal-based hardeners—when released into waterways or soil. It doesn’t bioaccumulate or persist as long as heavy metals or halogenated organics. That said, too much optimism neglects the fact that any novel compound transforms in nature, and its breakdown products need attention. Responsible manufacturers run extra waste treatment steps before discharge. Unfortunately, not all facilities stick to these best practices, especially where oversight is weak.
People deserve clear information about what goes into their workspace, their home, or their kid’s toys. I’ve seen that the companies committed to REACH or EPA disclosure tend to invest in safer chemistry, better ventilation, and ongoing monitoring. Harmless on paper doesn’t always guarantee safe in reality.
Fact-based transparency with up-to-date toxicology matters for everyone. Anyone using sebacic dihydrazide in a professional setting benefits from staying up to date with safety data sheets, proper ventilation, and personal protection. Regulatory agencies should keep requesting long-term and independent studies as industrial chemistry evolves, so we stop mistakes before they become public health issues.
Having spent years working with specialty chemicals, I’ve seen firsthand that storage and handling routines make up the backbone of chemical safety. Sebacic dihydrazide, used in epoxy curing and polymer modification, often comes up in conversations around safe practice for a good reason. This white powder looks innocuous, but without thoughtful protocols, things can go sideways quickly.
Sebacic dihydrazide isn’t volatile, so inhalation risk isn’t front and center, but dust still poses a problem. I recall a colleague in a poorly ventilated storeroom developing skin irritation after handling a shipment, just from trace powder contaminating the air and settling onto exposed skin. Dermal and respiratory exposure may sound minor, but regular contact raises cumulative risk. According to the European Chemicals Agency, direct contact can trigger allergic reactions and dermatitis, while long-term inhalation can affect sensitive individuals.
I’ve watched new techs think that keeping sebacic dihydrazide in any sealed jar means the job’s done. The truth is, this chemical absorbs moisture. If you throw it into a humid environment, you’ll be fighting caking and degraded material next time you scoop some out. Manufacturers and regulatory guidelines both highlight the importance of dry, cool storage conditions. That means full climate control, sealed containers—preferably lined or made from HDPE to ward off slow corrosion and leakage—and clear labeling.
Nobody looks forward to a spill, but a container drop can happen to anybody. Sweeping this stuff with a broom sends dust airborne. Suitable spill cleanup includes high-particulate respirator masks and washing down surfaces with copious water. Every technician who handles powder-form reagents should have training in this type of cleanup. Mixing it up with incompatible substances, especially oxidizers, is asking for trouble, so I always double-check neighboring shelf contents before new stock arrives.
Poor labeling sits behind half the near-misses I’ve seen in labs. Swapping containers happens, especially in busy stockrooms, so every jar of sebacic dihydrazide should have fresh, visible dates and warning stickers. It’s amazing how often a quick check stops someone from mixing old batches or grabbing the wrong powder entirely. I’ve watched companies use digital tracking systems that log every removal and restock; this kind of accountability means problems get caught fast.
Even the best sealed storage system can’t compensate for untrained staff. In my experience, putting knowledgeable people in charge does more to prevent accidents than investing in yet another layer of automated reminders. Annual in-person safety refreshers do wonders. Technicians should wear disposable gloves, protective goggles, and dust-resistant lab coats. Any sign of skin irritation means switching to thicker gloves or an alternative protective barrier.
Companies improve chemical safety culture by making small steps routine. Sharing incident reports, hosting regular discussions about close calls, and rewarding those who spot new hazards all help. By treating sebacic dihydrazide—or any fine powder—with the respect it deserves, labs stay productive, staff stay healthy, and costly mistakes stay rare. Anyone settling for less is rolling the dice with safety.
Factories and labs turn to sebacic dihydrazide for pretty specific jobs. This white, powdery chemical pulls its weight in several corners of manufacturing, especially where the final product gets put to the test both by people and by the weather.
The coatings business relies on additives that keep paint, varnish, or powder coatings from cracking and peeling. Sebacic dihydrazide steps up as a solid curing agent for epoxy and polyurethane systems. Its structure helps build tough networks inside the coating, making it less likely to chip or fade over time. Take a stroll through any city, and the buses, trains, and traffic signs might well owe their bright colors to chemicals like this one. Paint companies value its chemical stability and resistance to ultraviolet light, which adds years to painted surfaces.
Gluing things together isn’t just an art for crafters and carpenters; large-scale manufacturers also need adhesives that keep their grip through rough conditions. On a personal level, I’ve seen how fluctuating Chicago weather turns many tapes and glues into useless threads by winter’s end. Sebacic dihydrazide appears on ingredient lists for adhesives aiming to bond metals, plastics, or composites—especially those exposed to mechanical stress or temperature swings. It lends flexibility and keeps adhesive joints from turning brittle or delaminating during a freeze-thaw cycle.
Flexible plastics don’t just come from the raw resins themselves. Factories mix in a range of additives, and sebacic dihydrazide often finds a role here. In polymer production, it plays a part in cross-linking—basically tying long plastic molecules together to keep them from sliding past each other. Products from floor tiles to cable insulation get their strength in part from this and similar agents.
The pharmaceutical industry values any ingredient that can boost stability or function. Sebacic dihydrazide forms part of controlled-release matrices in drug delivery systems. Instead of releasing all the medicine at once, these matrices let the drug seep out slowly, extending its effect. Having worked with a neighbor who depends on medication with time-release features, I can see the difference it makes: fewer pills, fewer spikes, and steadier relief over a full day.
Experience in industrial settings taught me that every benefit comes with a requirement for care. Sebacic dihydrazide does not escape this rule. Handling raw powder means protective gloves, eye shields, and well-ventilated workspaces. Documented testing shows low acute toxicity, but any fine powder, if inhaled or left on the skin, brings its own set of risks. Proper training and storage keep incidents rare.
Innovation depends on materials that can adapt to new challenges. Researchers keep studying the use of sebacic dihydrazide as a building block for advanced hydrogels, eco-friendly plastics, and even biomedical devices. If companies invest in greener chemistry and transparent reporting, trust grows, and regulators find fewer reasons to block adoption. Consistent quality checks and clear safety labeling help move both worker health and end-user confidence in the right direction.
Industry grows by learning from both success and failure. Including sebacic dihydrazide in production lines can improve performance, but it shines brightest where staff understand the material and respect its limits. Regular training and faster reporting of near-misses close the loop, keeping everyone safer while giving finished products a longer, more reliable life.
| Names | |
| Preferred IUPAC name | decanedihydrazide |
| Other names |
Decanedioic dihydrazide
Sebacic acid dihydrazide 1,10-decanedicarboxylic acid dihydrazide |
| Pronunciation | /sɪˈbæsɪk daɪˈhaɪdrəzaɪd/ |
| Identifiers | |
| CAS Number | 4080-98-2 |
| Beilstein Reference | 2920806 |
| ChEBI | CHEBI:38664 |
| ChEMBL | CHEMBL3189278 |
| ChemSpider | 21050 |
| DrugBank | DB13904 |
| ECHA InfoCard | ECHA InfoCard: 100.045.344 |
| EC Number | 206-114-9 |
| Gmelin Reference | 104524 |
| KEGG | C18643 |
| MeSH | D003658 |
| PubChem CID | 72985 |
| RTECS number | VS2275000 |
| UNII | U70M63S9ER |
| UN number | UN2575 |
| Properties | |
| Chemical formula | C10H22N4O2 |
| Molar mass | 230.29 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.14 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | -1.0 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 10.68 |
| Basicity (pKb) | pKb ≈ 3.6 |
| Refractive index (nD) | 1.570 |
| Viscosity | 600 mPa·s (at 80°C) |
| Dipole moment | 2.92 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 356.4 J·mol⁻¹·K⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07,GHS05 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P261, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Autoignition temperature | 310°C |
| LD50 (median dose) | LD50 (median dose) of sebacic dihydrazide: >5000 mg/kg (oral, rat) |
| NIOSH | NJ2100000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.02 mg/L |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
Adipic dihydrazide
Azelaic dihydrazide Suberic dihydrazide Malonic dihydrazide Glutaric dihydrazide |