
Chemists first paid attention to malonic acid dihydrazide in the hunt for practical intermediates in organic synthesis. In early work from the first half of the twentieth century, research groups looked for new hydrazide derivatives that could provide both safety and reliability over volatile hydrazine itself. With the rise of industrial explosives and rocket propellants, the need for precise, stable hydrazides found malonic acid dihydrazide answering the call. Over decades, patent filings and scientific literature have detailed its use, sometimes quietly, as both a building block for specialty compounds and as a useful laboratory reagent. The substance’s twin hydrazide groups connected to a central malonic backbone remain useful today in the synthesis of more complex heterocycles and as a route for introducing nitrogen groups.
Malonic acid dihydrazide, known by many as N,N'-hydrazinedicarbonamide, stands out for its stable crystalline form and its capacity to serve both industry and research settings. With white to off-white granules, this compound resists the kind of quick decomposition that plagues similar molecules. Labs working with energetic materials, pharmaceutical intermediates, and specialty plastics often stock this as a go-to for introducing both carbonyl and hydrazide functionality. Its ability to act as a bridge in multi-step chemical syntheses, especially those pursuing nitrogen-rich rings, keeps it relevant on crowded laboratory shelves.
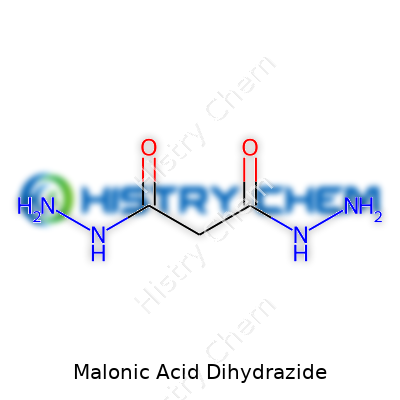
Malonic acid dihydrazide usually presents itself as a fine, odorless powder. Its molecular formula, C3H8N4O2, gives a clear indication of why it fits so well in nitrogen chemistry—it carries two NH-NH2 groups bonded to a central, simple carbon skeleton. Water solubility can be described as moderate. The compound doesn’t melt until exposed to relatively significant heat, decomposing before boiling, which hints at a lattice strengthened by hydrogen bonding and simple packing. Its chemical stability makes it easy to handle in most routine synthesis work, and its structure makes it less likely to evolve dangerous fumes at room temperature, as long as you respect the usual safety protocols.
Technical data sheets usually specify purity at or above 98%, with low levels of residual solvent and minimal heavy metal contamination. Most suppliers guarantee moisture content under 0.5%. Laboratories tally batch numbers and record storage temperatures, almost always advising a tightly sealed container at room temperature, away from direct sunlight and sources of ignition. The best product labeling gives hazard pictograms in line with the latest GHS standards: an exclamation mark for acute toxicity and a warning note about possible harm following skin or eye contact. Manufacturers who export beyond regional markets follow international shipment codes, labeling each container with both UN identifiers and local translation sheets.
Preparation generally starts with malonic acid or one of its derivatives. By reacting diethyl malonate with hydrazine hydrate, chemists selectively create the dihydrazide while controlling temperature just beneath the solvent’s boiling point. The process generates a fair amount of heat, so anyone scaling up production needs cooling jackets and efficient condensing columns. After separating the product from aqueous mother liquors, the crude dihydrazide gets washed and recrystallized from ethanol. Most published procedures emphasize slow addition and patient cooling to maximize yield and cut down on unwanted side products. In my own work, small tweaks to solvent ratios have turned out to be the difference between gummy sediments and solid, filterable product.
The twin hydrazide groups open the door to a wide range of chemical transformations. They react with aldehydes and ketones to form hydrazones and can cyclize under gentle heating to build up nitrogen-rich ring structures. Malonic acid dihydrazide also sees use in synthesizing triazoles and tetrazoles—scaffoldings for many modern crop protectants and pharmaceuticals. Curious chemists have played with retaining one hydrazide group and modifying the other, giving rise to tailored reagents for stepwise derivatizations. In the lab, this means you can convert an otherwise simple linear molecule into one that boasts new reactivity or binding capacity, expanding options for both acrylic resins and active pharmaceutical ingredients.
Over years and across trade catalogs, malonic acid dihydrazide has picked up an impressive list of aliases: N,N'-Dihydrazinylpropanedioamide, Dihydrazide of malonic acid, Malonyl dihydrazide, and N,N'-Hydrazinedicarboxamide, to name just a few. Each speaks to the same core of carbon, oxygen, and nitrogen. Whether a scientist is ordering a fresh bottle from a supplier in Europe or checking a reference in a Chinese language database, recognizing these names eases communication and streamlines procurement. In lab notebooks and patent applications, precise naming prevents confusion with acetyl, oxalyl, or isophthalic analogs.
Malonic acid dihydrazide deserves respect in every step from delivery to disposal. Direct skin exposure sometimes brings mild irritation, so gloves always stay between compound and skin. Dust inhalation can trigger both local and systemic effects: GHS labels and most material safety data sheets warn of the need for a fume hood and well-ventilated workspaces. No one in a responsible laboratory forgoes splash goggles and lab coats. In the case of accidental spills, simple house cleaning routines can sweep up dry solids, but anything in solution requires all spills to be flushed with copious amounts of water, followed by neutralization of residual hydrazine. Venting and waste storage procedures both underscore the same point—people, not just products, shape the safety picture.
The pharmaceutical sector values malonic acid dihydrazide as a precursor for heterocyclic ring syntheses, helping researchers chase new leads in antiviral and antitumor programs. Companies synthesizing specialty plastics and polymers use it to introduce precise reactivity into monomers destined for high-modulus or fire-resistant composites. Development programs in agrochemicals tap into its ease of transformation, using small-scale screenings to generate new triazole- or pyridazine-based molecules. Universities benefit too: for teaching labs, malonic acid dihydrazide shows up in classic hydrazone formation reactions, giving students hands-on experience in condensation chemistry. In explosive and propellant research, it supplies nitrogen in a controllable, less hazardous form than pure hydrazine, supporting the design of new gas-generating materials.
Ongoing research hasn’t left this compound behind; synthetic chemists still publish new routes for derivatives that branch off from the classic dihydrazide structure. In recent years, focus has shifted toward green syntheses—using less wasteful reagents, milder conditions, and even bio-derived starting materials. Work around the world investigates how malonic acid dihydrazide fits into solid-state reactions, microwave-assisted flows, or solventless procedures. I have seen new patents that rely on this compound to initiate tandem reaction cascades, shortening manufacturing timelines for complex active molecules. By publishing full spectral data and reproducible protocols, progressive research teams help new generations avoid the pitfalls of earlier, less safe practices.
Toxicity work on malonic acid dihydrazide reveals a moderate risk profile compared to more dangerous hydrazides. Animal studies suggest its oral LD50 falls in the high hundreds of milligrams per kilogram. At workplace levels, chronic exposure brings concern for both liver and kidney function, so regular monitoring, good personal protective equipment, and clear hazard labeling stay non-negotiable. Regulatory bodies in the US, Europe, and parts of Asia continue to step up scrutiny, linking sustained contact with possible sensitization or longer-term carcinogenic potentials. Institutional safety committees now demand full disclosure in grant reports and experimental planning, moving beyond the era of handwritten labels and ad hoc waste jugs. For researchers and production workers alike, the message is clear: respect, don’t fear, but never shortcut healthy habits.
Industrial innovation shows no sign of leaving malonic acid dihydrazide behind. Efforts to design cleaner, modular synthesis pipelines see this intermediary fitting easily into plug-and-play chemical reactors. In pharmaceuticals, regulatory push for safer, more environmentally friendly raw materials encourages formulators to look for compounds like malonic acid dihydrazide, where structural predictability can translate into reduced downstream hazards. Researchers working in sustainable synthesis now match this chemical with green solvents and continuous processing. Its history stands as a lesson in patience and problem-solving—by drawing from decades of hard-won lab experience, the next wave of chemists can make even more from this simple but powerful molecule. In ongoing work, cross-disciplinary teams continue searching for new modifications and uses, pushing malonic acid dihydrazide beyond legacy status toward a future shaped by both need and creativity.
Malonic acid dihydrazide stands out in the world of organic compounds, especially for chemists who work with hydrazide groups and carbon skeletons. Its chemical formula is C3H8N4O2. This structure gives it a backbone that carries both hydrazide functional groups flanking the methylene center. That setup actually comes from hydrazine’s reaction with malonic acid, which creates a compound useful in research, especially when looking at reactivity and synthesis.
Sitting at the crossroads of practical chemistry and research, malonic acid dihydrazide is a go-to in the lab for those who work on ligands, metal complexes, and organic frameworks. Lab experience shows chemists count on its predictable behavior—something not always easy to find. I’ve worked through long syntheses where stability and reactivity must be balanced. Malonic acid dihydrazide accomplishes this, serving as a linking unit for larger molecules or as a building block for engineering new materials.
Its two hydrazide groups grant it ability to bind metals and organic partners, important for design of pharmaceuticals and catalysts. There’s a story to tell here: many cancer drugs and agricultural chemicals trace synthesis steps back to simple core compounds like this one. Chemists appreciate the value in its C3 backbone and the symmetry it provides in reactions involving coordination compounds and even in crystal engineering.
Work in the lab or industry, and you see how properties matter. Malonic acid dihydrazide is a white crystalline powder. It dissolves in water and polar solvents, making it adaptable. Its melting point sits around 178–182°C. Toxicology data stay sparse, though it’s clear from experiment and experience that gloves and proper ventilation are wise.
Looking into literature reviews, its use continues to expand. Not only do academic groups work with it, but so do teams researching polymers and coordination chemistry. As of last year, more papers reference malonic acid dihydrazide than a decade ago. This matches trends seen in medicinal chemistry, where functionalized hydrazides serve as key intermediates.
One problem crops up in hazards, especially with hydrazine derivatives. Exposure to hydrazine-related materials poses risks for skin sensitization and, at higher doses, even more severe outcomes. Better safeguards in academic laboratories, more prominent safety data sheets, and practical training can make a real difference here. It’s not uncommon to see young students underestimating risk because the powder looks harmless. Safety culture can't be just a sign on a wall. It grows from experience, stories told, and real examples.
Supply chain reliability also pops up as a problem. Specialty chemicals like this sometimes get caught in logistics bottlenecks. Partnering with reputable chemical suppliers, robust record-keeping, and collaborative networks among labs can help ensure research projects don’t stall out. Another promising option: local synthesis on demand for core intermediates.
Researchers continue to push new uses for malonic acid dihydrazide. Rapid growth areas include new materials that trap metal ions for cleanup and targeted drug synthesis. Sharing open, reliable data and strengthening community networks supports transparency and quality. Real progress travels hand in hand with hands-on skill, respect for materials, and a willingness to share what works.
Malonic Acid Dihydrazide isn’t a household name, but it plays a silent yet crucial role in several fields. With a structure related to hydrazine, this compound brings a reactive edge that has caught the attention of scientists and engineers who want both versatility and dependability. Spending some years talking with folks in chemical manufacturing and reading peer-reviewed publications can reveal just how much work a single molecule can put in across industries.
People in defense production recognize Malonic Acid Dihydrazide as a building block for powerful rocket fuels and explosives. Some energetic materials reach their punch by adding this compound. Its nitrogen-rich structure delivers high energy output, making it a candidate for gas-generating agents in propellants. Growing up in a family with military engineers, conversations around these applications often focused on handling safety and storage. News stories from the 1980s described research projects that cut hazards and improved stability for safer storage of fuels that would otherwise pose big risks.
Scientists aim for innovation and reliability in drug development. Manufacturers use Malonic Acid Dihydrazide to build complex pharmaceutical compounds. Its ability to form strong chemical bonds helps researchers assemble molecules with specific biological activity. Academic papers, especially from India and Japan, highlight uses as a coupling reagent or a starting material for various pharmaceuticals and analytical agents. Such direct roles help researchers cut down synthesis time and improve the purity of end products.
Malonic Acid Dihydrazide finds its way into the making of specialty polymers. Research teams in Europe have shared how this compound introduces crosslinking in polymer chains, creating coatings with unique properties like improved water resistance or enhanced flexibility. From experience with material science students, I’ve watched lab tests where small tweaks to a polymer’s backbone—sometimes with just a pinch of this acid dihydrazide—changed the entire outcome of the finished product.
Laboratories often turn to strong chelating agents for metal ion detection. Malonic Acid Dihydrazide binds selectively to certain metal ions, acting as a stabilizer in trace-level measurements. During an internship with an environmental testing center, I watched professionals trust this compound for reliable detection of pollutants in drinking water. The sensitivity and reliability offered by such reagents have direct health implications, driving better regulations and monitoring.
Widespread use of Malonic Acid Dihydrazide doesn’t escape challenges. Handling and storage require strict safety protocols due to possible toxic decomposition products. Some manufacturers look for greener production routes, limiting environmental impacts. Progressive companies redesign their processes using renewable feedstocks or developing recycling methods for waste minimization. The chemical research community encourages open dialogue about safer alternatives and tighter workplace regulations, especially in developing countries with rapidly expanding chemical sectors.
Across explosives, pharmaceuticals, polymers, and analytical labs, Malonic Acid Dihydrazide quietly keeps industry moving forward. Its story reflects the sometimes invisible backbone of progress: a stable molecule, used with care, can make front-page news possible even if no one outside the lab ever learns its name.
Malonic acid dihydrazide doesn’t make headlines, but in a chemistry lab, respect for this powder means fewer unwanted surprises. I’ve spent enough years around chemicals to know that simple steps protect people far more than false confidence. In my own work, ignoring the guidance around lab basics—gloves, eye protection, proper ventilation—has always come back to bite someone on the team. Malonic acid dihydrazide is no exception; in fact, it reminds us every bad lab tale begins with “I thought it wouldn’t happen to me.”
People ask if malonic acid dihydrazide is “dangerous.” Here’s the honest answer: if you treat it like powdered sugar, you’re putting your lungs and eyes at risk. Fine particles get airborne and land in places they shouldn’t. Exposure may irritate the eyes, skin, and respiratory system. Safety Data Sheets make it clear—skin contact and inhalation are the biggest issues here. Too many forget that handling any hydrazide compound can trigger allergies or worse with enough mishandling. I’ve seen coworkers dismiss PPE during high-pressure days, and it always leads to rushed cleanup and regret.
Many new chemists assume gloves and goggles are enough. That’s a start but not the end. A properly fitted lab coat, splash-proof goggles, and disposable gloves form your first layer of defense. Never pour or handle this chemical outside of a chemical fume hood, even for a quick transfer. Good air flow clears away fumes and dust, which keeps your lungs functioning the way you want them to. Those old stories about small exposures "not mattering" haven’t aged well. Medical journals, including detailed hydrazide-related studies, link repeated exposure with chronic irritation or long-term health complications.
Safe storage means controlling humidity, temperature, and access. Don’t leave containers on open shelves next to acids, bases, or food. One careless moment with the wrong jar can spark a spill or, worse, a slow chemical reaction that nobody notices until the smell changes. In my labs, I keep it in tightly sealed, labeled containers inside a dedicated cabinet away from sunlight and clear from anything reactive.
Never let waste disposal fall through the cracks: that’s how labs attract uninvited inspections. Liquid and solid residues belong in properly marked hazardous waste bins, never in the regular trash or down the drain. If you’re unsure about waste protocols, talk to your Environmental Health and Safety (EHS) officer before starting the experiment.
A culture of safety saves more than just regulatory headaches. In busy labs I’ve managed, clear signage, quarterly safety retrainings, and a shared attitude of “never cut corners” built habits that protected everyone, from students to senior researchers. Each new protocol update comes from last year’s mistakes. Peer-reviewed reports back up every decision: treating potentially hazardous organic compounds with respect saves careers and lives.
There’s no need to invent complicated procedures. Relying on established best practices for equipment, personal safety gear, ventilation, and secure storage takes most of the danger out of working with malonic acid dihydrazide. For all of us with science careers or personal interest in safe handling, staying a little humble beats wishing we’d taken safety more seriously.
Malonic Acid Dihydrazide lands in the spotlight from time to time, especially in fields like materials research and fine chemistry. Many chemists have run up against the practical question of its solubility before ever thinking about its use in synthesis or formulation. The way a compound dissolves—or fails to dissolve—can make or break a project. Years back, I remember struggling to coax poorly soluble compounds into solution for basic testing. The right solvent can feel like unlocking a door to progress.
Malonic Acid Dihydrazide carries the formula C3H8N4O2. Structurally, it has two hydrazide groups attached to a malonic acid core. This chemical features several nitrogen and oxygen atoms, so it forms a network of hydrogen bonds both within itself and with surrounding molecules. Multiple sources—peer-reviewed literature, supplier technical data, and decades of commercial practice—all confirm a central point: malonic acid dihydrazide dissolves well in water. In fact, its polar nature leads to ready mixing with water at room temperature. Researchers working in aqueous systems, including those working on crystal growth or spintronic precursors, see this solubility as a clear advantage.
The story gets interesting when you switch to common organic solvents. Chemistry handbooks and supplier technical sheets report low to negligible solubility in ethanol, acetone, ether, and most non-polar or moderately polar solvents. That means labs using these as their go-to solvents often have to pivot to water or work with suspensions—adding in more steps for removal, washing, or extraction. I’ve found that testing in DMSO or DMF can sometimes pull off limited dissolution, but nothing matches its behavior in water. This peculiarity shapes which projects can use malonic acid dihydrazide.
If a chemist wants to incorporate malonic acid dihydrazide in a reaction or preparation, they need to plan around these facts. Water-based synthesis lines up well for its use. In biotech or pharmaceuticals, water compatibility streamlines processes and helps avoid complicated solvent exchanges. Environmental safety benefits as well, since fewer toxic organics get involved. On the flip side, folks working in organic media scramble to find workarounds, often choosing different reagents or designing multi-step methods. Sometimes, limited compatibility with solvents blurs the line between discovery and frustration. In my experience, a promising compound with poor solubility usually leads to tough decisions—delay work or change direction.
Solubility issues invite creative problem-solving. For malonic acid dihydrazide, emulsifiers or co-solvent systems rarely do the trick, since its strong hydrogen bonding demands polar, protic environments. Water remains the gold standard, but improvements could come from working on derivatives or salts with more favorable properties. Advances in nano-dispersion and solid-phase chemistry also offer new angles—though not all labs have the luxury of this technology yet. To truly open up possibilities, transparent data reporting is key. Users deserve up-to-date information from vendors and researchers. Reliable CAS data, supplier specs, and academic reports help prevent wasted effort and drive more effective planning.
Understanding solubility isn’t a box to tick; it shapes projects from early feasibility through to the final product. Malonic acid dihydrazide plays nicely with water but turns stubborn in most other solvents. Planning around this reality allows chemists to sidestep obstacles and tap into its chemical potential—without losing nights over trial-and-error in the solvent cupboard.
Ask anyone who’s worked in a lab for a while—chemical storage can eat up more hours than planning the actual experiments. Malonic acid dihydrazide sits among those compounds that demand a bit of respect in storage. My experience tells me it pays dividends to go the extra mile with preservation, both for safety and for keeping a product you can count on. No one likes watching a key reagent lose effectiveness long before the expiration date.
Heat rarely does chemicals any favors, and malonic acid dihydrazide proves that point. Room temperature seems fine for a coffee break, but it invites hydrolysis and decomposition in sensitive solids. To avoid those slow, sneaky changes, keep the temperature consistently below 25°C. If you manage a storeroom, putting this one onto lower shelves, away from direct heat sources or sunlight, usually works best. Fridges have saved researchers more often than we’d like to admit, especially in summer. Fluctuating temperatures speed up chemical breakdown; so steady chill earns our trust.
Once, I left a poorly sealed bottle near a washing sink—cost me a weekend’s work when the batch stopped performing as advertised. Malonic acid dihydrazide, like many hydrazide compounds, absorbs water from the air. That moisture kicks off hydrolysis and turns powdered reagent into an unworkable mess. Always use airtight containers—screw caps with liners beat old cork stoppers hands down—and stash a fresh desiccant pack inside each bottle. In one lab, we started adding a silica gel pack before closing the lid; it was a low-cost tactic with instant payoffs in shelf life.
Some lab veterans store malonic acid dihydrazide in amber bottles, even though it’s not highly photosensitive. Light doesn’t break it down at a frightening pace, but keeping containers away from windows and fluorescent bulbs helps slow degradation and limits unwanted impurities sneaking in. Opening a jar as infrequently as possible—and avoiding cross-contamination by switching spatulas from other chemicals—makes a big difference. One contaminated batch can dump a project for a whole research group.
It’s hard to overstate the value of clear labeling and sensible isolation. I’ve seen too many overlooked bottles cause confusion when staff changes shift. Each bottle needs a conspicuous label with both the full chemical name and date of receipt. Store it on a separate shelf from acids, bases, or potent oxidizers. Hydrazides mix poorly with many other laboratory staples; one missed alphabet order can set up a dangerous interaction just waiting to happen.
Over the years, a few habits have proven effective: Keep containers tightly sealed, add a fresh desiccant, store below 25°C and away from light, mark every container with clear, current info, and double-check you’re not stacking it close to reactive neighbors. In shared spaces, communicating these expectations in training sessions helps new team members learn fast and avoid simple storage errors.
Good storage has more impact than a lot of folks realize. Maintaining stable ingredients drives solid, repeatable results—saving money, time, and keeping people safe. Following simple, proven storage advice turns malonic acid dihydrazide from a risk into a reliable reagent. Experience reinforces one truth: respect the storage, and lab life gets easier.
| Names | |
| Preferred IUPAC name | malonohydrazide |
| Other names |
MAH
malonyl dihydrazide propanedihydrazide malonylhydrazide malonic acid dihydrazide malonic dihydrazide |
| Pronunciation | /maˈlɒnɪk ˈæsɪd daɪhaɪˈdreɪd/ |
| Identifiers | |
| CAS Number | 38150-37-3 |
| Beilstein Reference | Beilstein 1720240 |
| ChEBI | CHEBI:52280 |
| ChEMBL | CHEMBL16656 |
| ChemSpider | 21865952 |
| DrugBank | DB14626 |
| ECHA InfoCard | 100.016.900 |
| EC Number | 2517-34-2 |
| Gmelin Reference | 76697 |
| KEGG | C14355 |
| MeSH | D008288 |
| PubChem CID | 14814 |
| RTECS number | UU9625000 |
| UNII | 86QG2G686T |
| UN number | Not regulated |
| CompTox Dashboard (EPA) | DTXSID7054212 |
| Properties | |
| Chemical formula | C3H8N4O2 |
| Molar mass | 134.12 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | Odorless |
| Density | 1.62 g/cm³ |
| Solubility in water | soluble |
| log P | -2.12 |
| Acidity (pKa) | 2.5 |
| Basicity (pKb) | 10.10 |
| Magnetic susceptibility (χ) | -43.0 x 10^-6 cm³/mol |
| Refractive index (nD) | 1.670 |
| Dipole moment | 5.13 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 231.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -143.23 kJ/mol |
| Pharmacology | |
| ATC code | This product does not have an ATC code. |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): >5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5000 mg/kg (rat, oral) |
| NIOSH | Not Established |
| PEL (Permissible) | Not established |
| REL (Recommended) | 4.5-7.0 |
| Related compounds | |
| Related compounds |
Malonic acid
Malonic acid diethyl ester Malonamide Succinic acid dihydrazide Oxalic acid dihydrazide Adipic acid dihydrazide |