
Diethyl succinate has a history reaching back to the early industrial days of organic chemistry, when researchers began unlocking new routes for synthesis and exploring the commercial value of esters. Chemists figured out that reacting succinic acid with ethanol, using acid catalysts, produced a colorless, oily liquid with fragrant notes. Through the decades, its presence grew in both laboratory and industrial environments. The growth of polyester and resin industries underscored its relevance. By the twentieth century, improvements in distillation and esterification helped companies reliably produce diethyl succinate at scale, bringing down costs and ensuring purer outputs. Its historical evolution mirrors broader shifts in chemical production, where efficiency, output, and purity keep advancing in sync with demand.
Diethyl succinate stands out as a versatile ester with a mild, slightly fruity scent. Chemically recognized as the diethyl ester of succinic acid, it comes in handy for a wide array of uses, from intermediate steps in chemical synthesis to everyday consumer flavors and fragrances. In my experience, even a modest laboratory setup can use it in place of more expensive esters when making certain drug intermediates. Producers typically supply it as a clear, colorless, oily liquid, lending a broad working range for both chemists and manufacturers.
Diethyl succinate carries the molecular formula C8H14O4 and has a molecular weight around 174.2 g/mol. In the lab, it boils just above 215°C and melts a little below -20°C. Its density is close to 1.07 g/cm³, and it mixes freely with common organic solvents but barely with water. Its gentle odor sometimes hints at apples or mild alcohol. The compound withstands moderate heat, but strong acids or bases accelerate its breakdown. This stability serves well for procedures that involve gentle heating, letting researchers and manufacturers use it with little trouble over a wide temperature range.
On chemical labels, bottles usually list purity, commonly 98% or higher for commercial batches. Container data includes identification numbers, hazard warnings, dates of manufacture, and storage guidelines, which often recommend cool, dry spaces away from oxidizers. Safety sheets highlight flammability—the flash point hovers near 90°C—and recommend proper ventilation for handling. Pipes and containers in factory setups use durable seals and suitable linings to contain both vapor and liquid forms, avoiding corrosion and leaks. My own lab received drums rated for transport under UN 1993, following all legal and industry standards for hazardous liquids.
Most commercial production relies on Fischer esterification. Succinic acid reacts with ethanol in the presence of strong acids like sulfuric acid, driving the reaction under reflux. Removing water shifts the balance toward more ester production. Afterward, the crude mixture gets neutralized, washed, and purified by distillation. Larger factories sometimes favor continuous processes that use column reactors and automated separation units. In research setups, condensation and ethanol removal under reduced pressure can help prevent thermal degradation, boosting yield and cutting side reactions. These methods reflect the broad range of equipment and resources found in different production environments.
Diethyl succinate offers more than simple ester functionality. In organic synthesis, it serves as a building block for alkylation, condensation, and hydrolysis reactions. The two ester groups provide symmetry, which strong bases like sodium ethoxide exploit to form enolates—the starting point for Knoevenagel or Claisen-type condensations. Chemists can convert it back to succinic acid or transform it into amides, anhydrides, or diols, all through practical and manageable steps. In medicinal chemistry, functionalizing the molecule with ring-closing or chain-extension reactions often creates compounds with interesting bioactivity. These modifications matter in both small exploratory labs and major manufacturing outfits.
Diethyl butanedioate, butanedioic acid diethyl ester, and succinic acid diethyl ester all show up in catalogues and regulatory documents for this compound. These alternate names sometimes confuse those new to ester chemistry, but each term points to the same structure and set of properties. In supply chains, barcodes and catalog codes often supplement names to prevent mix-ups. For anyone ordering from international sources, reviewing synonyms can avoid costly shipment mistakes.
Managing diethyl succinate in industrial and laboratory environments calls for attention to both acute and chronic exposures. The liquid evaporates at room temperature, and long-term inhalation of vapor may irritate eyes and airways. Splashes on skin or into eyes cause mild but persistent irritation unless washed quickly. Certified gloves, eye shields, and lab coats form the backbone of personal protection. Storage in flammable-liquid-approved containers stands as a routine measure. Training staff on spill management and emergency protocols keeps risks low. Across facilities, compliance with OSHA, REACH, and relevant GHS guidelines offers a legal baseline for safety, but ongoing vigilance and clear instructions make the critical difference.
Diethyl succinate makes an impact in many sectors beyond basic chemical production. In the flavor and fragrance world, its mild scent adds complexity to fruity blends, especially for baked goods and candy. Pharmaceutical companies use it as an intermediate, preparing barbiturates and other synthons that benefit from symmetrical carbon backbones. Its abilities as a plasticizer find value in producing flexible polymers, resins, and some coatings. In specialty solvents, it dissolves polar and nonpolar compounds, making it useful for both research and industrial cleaning. Over the years, I’ve seen startups use it as a green chemistry alternative for safer solvent blends, furthering sustainability claims as regulations tighten globally.
Ongoing innovation draws inspiration from environmental, economic, and regulatory pressures. Scientists keep exploring new ways to synthesize diethyl succinate at lower temperature or with bio-based ethanol, hoping to reduce greenhouse gas footprints and shrink costs. Catalysts now under development cut energy use and waste discharge, paving the way for greener industrial models. Universities and corporate labs experiment with the molecule’s derivatives, chasing opportunities for next-generation pharmaceuticals and biodegradable materials. Pilot projects explore using waste biomass as a source of succinic acid, tying chemical manufacturing into closed-loop resource streams. These efforts point to greater market penetration, fresh applications, and expanding global demand.
Available studies indicate moderate toxicity at high doses but low risk in most use scenarios, provided pods, vials, or drums receive careful handling. The compound breaks down in the body into ethanol and succinic acid, both substances naturally found in metabolism. Animal studies report minor respiratory and digestive irritation but no evidence of carcinogenic or mutagenic effects. Prolonged, concentrated exposure can stress kidneys and liver in rodents. Human workplace risks mainly relate to improper storage or disregard for ventilation and personal protection protocols. I’ve witnessed that all too common tendency to slip on gloves only after something splashes. Good training, quick reaction, and regular health screening keep operators healthy and reduce accident rates.
New approaches for making diethyl succinate from renewable resources change the economics of ester production. With a growing global push toward non-petrochemical feedstocks, biobased succinic acid unlocks cleaner syntheses, positioning diethyl succinate as a green alternative for plasticizer, solvent, and intermediate needs. Drug development teams rely on its backbone for new molecules that treat infections or metabolic disorders. As regulatory frameworks lighten the load on compounds with established low-toxicity records, more formulators can use diethyl succinate in food-contact and cosmetic products. Collaboration between academia and industry should further improve purification, introduce more reactive derivatives, and create safer, more stable blends for both consumer and industrial uses. The continuing story of diethyl succinate is one of broadening use, higher safety standards, and deeper integration into sustainable chemical practices.
If you take a closer look at labels in your kitchen or bathroom, you might run into complicated names like Diethyl Succinate. It’s not some new buzzword or a cutting-edge invention. Chemists started working with it decades ago, and it’s found its way into more corners of modern life than people realize. One of the main reasons is the fruity smell it brings to the table. Anyone who has opened up a bottle of flavored drink or a cosmetic item knows how much scent changes the whole experience. Diethyl Succinate brings sweet notes that remind people of candy or pineapple, so it shows up in flavorings and fragrances widely.
Think about walking down the perfume aisle and catching a whiff of something breezy. Chances are some ingredients inside come from families like succinates. Perfumers lean on Diethyl Succinate because it helps round out floral or fruity perfumes. It doesn’t overpower other notes. Instead, it provides a smooth and subtle undertone. You get a balanced fragrance with fewer synthetic harsh edges. It acts the same way for food flavoring. The FDA lists this compound as safe in tiny amounts. Bakers and drink-makers rely on it for jammy or ripe fruit notes. If you’ve ever tasted fruit spreads or pie fillings with an extra pop, Diethyl Succinate may have had a role.
Factories and laboratories count on Diethyl Succinate for more than just flavor. As a solvent, it dissolves other chemicals without leaving much behind, which appeals to folks concerned about safety. Paint and plastic makers sometimes select it because it breaks down cleanly. Chemists in green chemistry also appreciate that it comes from succinic acid, which biorefineries can produce from sugar or plant waste. Instead of turning only to petroleum sources, manufacturers can turn to more sustainable methods and reduce fossil fuel use.
Making medicine isn’t only about growing the right plants or isolating natural compounds. Drug designers use chemicals like Diethyl Succinate to build complex molecules. It helps kick-start reactions or links together smaller parts. This ability saves time and cuts down waste during manufacturing. Well-known antibiotics, vitamins, and lab reagents got their start using succinate derivatives. Decades of safe handling in medicinal chemistry offer peace of mind for professionals and patients alike.
Any discussion of chemicals hits a fork in the road—usefulness and safety. Agencies like the Environmental Protection Agency and the European Chemicals Agency keep tabs on studies and issue guidelines. Diethyl Succinate’s safety profile looks much better than harsher industrial solvents. Fragrance and food chemists work within approved limits to protect public health. In daily life, most people come into contact with such substances through tiny amounts that do not build up in the body.
New research looks at using biosuccinic acid to make not just flavors and fragrances, but biodegradable plastics and eco-friendly solvents. These efforts could shift demand away from petroleum and toward greener ways to make familiar products. It feels promising to see older chemicals take on new, cleaner jobs.
Walk down any grocery aisle, and you’ll spot ingredient names that don't roll off the tongue. Diethyl succinate is one of those. This chemical serves as a flavoring agent in some processed foods and drinks. Its slightly fruity, sweet aroma makes it attractive to food manufacturers looking for a boost in taste. You’ll also find diethyl succinate used in perfumes and sometimes even in pharmaceuticals.
Curiosity about what we eat never hurts, especially with chemicals involved. Diethyl succinate has been part of the food industry for decades. Regulatory bodies like the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA) have weighed in on its safety. According to the FDA, diethyl succinate falls under the "Generally Recognized As Safe" (GRAS) category when used in food at typical concentrations. Sounds reassuring at first glance, but food safety doesn’t exactly work on autopilot.
Research dating back decades hasn’t flagged significant safety concerns at common exposure levels. Studies where animals ingest large amounts over time showed low toxicity and didn’t reveal evidence of carcinogenicity or reproductive risks at doses humans would ever encounter through food. Still, those studies usually pay attention to basic health markers. Long-term, subtle effects can feel harder to untangle, especially when mixtures of food additives interact in ways nobody studied before.
My own approach to eating steers clear of trying to memorize every E-number or scientific label. Living with food allergies and intolerances has taught me to stay aware, not paranoid. Most folks don’t need to lose sleep over tiny amounts of diethyl succinate, but not everyone reacts the same way to food additives. Some people with chemical sensitivities might notice headaches, skin issues, or digestive troubles after consuming foods full of artificial flavorings. These cases are rare, though, and diethyl succinate doesn’t pop up on lists of common allergens.
Food safety regulations exist for a reason. They set clear limits after reviewing data from different studies and considering daily dietary patterns. The low levels allowed in food products matter more than whether diethyl succinate exists on a label in the first place. Overconsuming any processed foods—regardless of which compounds show up in the ingredients—can pile on health problems. Salt, sugar, preservatives, and flavor enhancers make up a cocktail with potential downsides far beyond one chemical alone.
Getting hung up on every single food additive makes eating stressful. Instead, making meals from scratch with whole ingredients puts the power back in your hands. Choosing fresh fruits, vegetables, whole grains, and minimizing packaged snacks keeps exposure to all sorts of unnecessary chemicals low. For those with rare sensitivities, keeping a food diary and checking labels helps pinpoint possible triggers. On a bigger scale, governments and regulators must keep updating safety reviews as new evidence comes in. Consumers can support transparency by buying from brands that clearly label ingredients and explain sourcing.
Diethyl succinate, like most food additives approved for use, presents little risk for the vast majority. Scientific oversight and regulations keep levels well below danger zones. People who stick mostly to whole and minimally processed foods avoid many unknowns—diethyl succinate included—without having to follow every new headline with alarm.
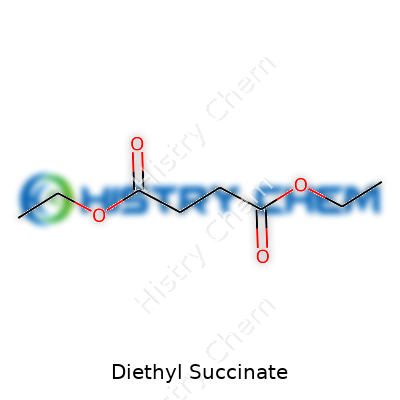
Diethyl succinate pops up in all kinds of places—chemistry labs, pharmaceutical catalogs, food manufacturers. It's easy to look at a formula and move on, but understanding what those letters and numbers represent makes a difference. Diethyl succinate has the formula C8H14O4. That tells you it holds eight carbon atoms, fourteen hydrogens, and four oxygen atoms. Each of these atoms plays a role in how this ester behaves. This formula shapes its physical form, boiling point, and how it smells and tastes.
Back in college organic chemistry, when a professor wrote C8H14O4 on the chalkboard, it barely registered. Later, while working on natural flavor design for a food company, the meaning clicked. Diethyl succinate tastes like rum and has a fruity aroma, and manufacturers use this stuff to mimic those flavors. If you see "naturally flavored" soda or candy with a mild sweetness, you’ve probably tasted it. The exact formula ensures quality control, safety, and label accuracy. Using the wrong ester—even one carbon off—can lead to very different outcomes, some of them not so pleasant for the consumer.
Pharmaceutical chemists use diethyl succinate as a building block. Its structure, based on that formula, allows the creation of more complex molecules for drugs or diagnostic agents. The formula dictates what reactions are possible, what byproducts show up, and how safe the process feels for researchers and end users. In one contract lab, a mix-up between diethyl succinate and ethyl succinate led to confusion—and extra costs. Clear chemical formulas, not just names, keep operations running smoothly and safely.
I once spoke with a quality manager at a chemical supplier who said, “One letter off in a formula, and you’re inviting trouble.” Reliable products depend on accurate chemical identities. For regulatory bodies like the FDA or EFSA, the formula points to properties, safety data, and legal compliance papers. Clients check certificates of analysis for C8H14O4 because they can’t take risks on batch variations or allergens sneaking in. Getting the formula right is not just scientific; it’s business-critical.
Errors in chemical labeling or formula entry cause accidents and wasted money. Standardizing record-keeping, using database checks, and providing regular training fought many of these problems at places where I worked. Digital inventory controls flagged discrepancies before a batch hit the mixer, catching typos that get missed by tired eyes. Cross-checking lot numbers with molecular formulas restored confidence among team members and reduced panic during audits.
Schools and workplaces could bridge gaps between textbook formulas and their applications. Training that connects the dots between a line on paper and the taste of a hard candy or the shelf life of a medicine builds respect for accuracy. People remember why a single letter matters after seeing the fallout from a miscalculation. The chemical formula of diethyl succinate—C8H14O4—is not just notation. It’s a pin at the intersection of safety, taste, and innovation.
Storing chemicals at work or home never feels like a casual task. There’s a difference between “it’s fine for now” and doing things right, especially with industrial-grade compounds such as diethyl succinate. Plenty of people overlook everyday risks in the name of convenience, but safety habits have a way of paying off. One mistake I saw during my grad school days was a cracked bottle of solvent sitting on a sunny window ledge – a close call that nearly turned into a bigger problem.
Diethyl succinate may not sound scary. It’s used as a flavoring agent, in organic synthesis, and sometimes in pharma. No alarms go off just hearing the name. Still, trouble creeps in when storage gets sloppy.
Chemicals love to break down quietly, and heat speeds up the process. Diethyl succinate does best out of direct sunlight and away from heat sources. Even if it doesn’t explode or catch fire on its own, sunlight and high temps can still nudge it along to form unwanted byproducts. I’ve seen more than one lab get stuck with a bottle of degraded reagent, only to wonder why an experiment fails and why a mysterious smell hangs in the air.
Simple shelves above a radiator or near a sunny window make easy mistakes. Keep diethyl succinate in a cool room, and always check for any strange change in color or appearance. Temperature drifts lower the shelf life quietly, ruining purity and introducing safety issues no one expects.
Diethyl succinate doesn’t play well with water or oxygen. Moisture can seep through threads in poorly sealed bottles, causing hydrolysis. That breaks down the compound, which means less reliable results and potential formation of unpleasant odors or acidic byproducts. Honest experience: the sharp sting in the nose from a forgotten bottle with a loose cap in a damp chemicals cabinet stays with you for years.
Tight, screw-capped glass containers help keep out humidity. Glass works better than plastic because certain plastics let vapors pass over time. Polyethylene containers, especially, have a reputation for subtle leaks — something I learned after chasing down mysterious smells in a teaching lab. Designate certain cabinets for organics like diethyl succinate, and keep those spaces clean and dry.
Don’t count on a memory to tell you what’s in an unmarked bottle. I can admit: plenty of times in shared labs, unlabelled bottles caused confusion and even panic. Proper storage always includes thorough labeling – chemical name, date opened, and source. It takes no big effort to clearly mark hazards and keep people in the loop about what they handle.
Set a calendar reminder to check chemical stocks quarterly. Inspect every bottle for a tight cap, signs of leaks, or anything odd. If something smells off or looks cloudy, it’s time to toss that batch with care and order fresh inventory.
Safe storage of diethyl succinate boils down to three habits: keep it cool, dry, and well-labeled. Glass bottles with firm seals in closed cabinets offer good protection. Don’t cut corners thinking certain chemicals are too mild to matter. Every time someone ignores a small leak or a faded label, it sets up trouble for later. Smart storage routines protect everyone – from the seasoned chemist to the intern just starting out.
Diethyl succinate shows up as a colorless liquid, carrying a faint, fruity scent that reminds some folks of ripe pears or sweet apples. You won’t spot it thick or syrupy in your beaker; it glides with about the same ease as many common organic solvents, with a viscosity that stays low enough to mix fast and pour smooth. It boils right around 218°C, a little above water's boiling point, adding some resilience for lab uses where higher temperatures come into play.
Pour diethyl succinate on your hands and you’ll notice it feels light, not sticky. Its density lands at about 1.07 grams per cubic centimeter at room temperature — just a touch heavier than water. Drop it in water, though, and you’ll quickly notice the two don’t want to mix. Diethyl succinate dissolves better in most organics like ethanol and ether. This lack of water solubility comes from its ester groups and a fairly extensive carbon framework, which resist hydrogen bonding with water molecules.
In the lab, knowing these physical traits means safer working conditions. A boiling point above 200°C keeps it intact under basic distillation or heating setups. Folks using it to synthesize medicines or plasticizers count on that stability to avoid dangerous decompositions. Just like ethanol, diethyl succinate works as a medium for reactions—chemists lean on its reliable, non-reactive nature with many reagents.
If you’ve worked in flavor or fragrance production, that fruity aroma probably rings a bell. Everyday items, from candies to perfumes, owe part of their character to this compound’s smell and liquid form, which makes it easy to blend and measure. In the food industry, it sometimes acts as a flavoring agent, working in very low concentrations to avoid overpowering everything else.
Since diethyl succinate evaporates more slowly than lighter solvents like acetone, spills linger and could stick to surfaces, demanding proper cleanup. Contact with open flames or hot surfaces, though, brings a risk. Its flash point—right around 91°C—sits lower than some might expect, meaning it can ignite if left near heat sources.
Release into soil or waterways carries its own set of worries. Plants and aquatic life don’t break down esters like diethyl succinate at the drop of a hat. Safe storage and responsible waste handling stay critical in every operation, not only to protect workers but also to prevent long-term buildup in the environment. My own time as a lab assistant drilled this lesson in fast—what evaporates out of bottles or drains can land right back in our ecosystem if we don’t take care.
Laboratories and factories can lower risk with simple steps. Strong ventilation stops vapors from building up. Like with all chemicals, the right gloves and goggles protect skin and eyes from irritation or burns. Clear, consistent labeling avoids mix-ups, especially when handling similar-looking esters.
Diethyl succinate highlights the huge value of paying attention to physical properties before handling any chemical, not just after an incident. Getting familiar with boiling points, density, and solubility means fewer surprises and a smoother experience whether you’re scaling up production or tinkering with a home experiment kit. Understanding these nuts-and-bolts details helps everybody—from students to seasoned chemists—work smarter, safer, and with more respect for the stuff around us.
| Names | |
| Preferred IUPAC name | Diethyl butanedioate |
| Other names |
Succinic acid diethyl ester
Diethyl butanedioate Butanedioic acid diethyl ester Ethyl succinate |
| Pronunciation | /daɪˈɛθ.ɪl səksˈɪn.eɪt/ |
| Identifiers | |
| CAS Number | 123-25-1 |
| Beilstein Reference | Beilstein Reference: 1741411 |
| ChEBI | CHEBI:35282 |
| ChEMBL | CHEMBL356140 |
| ChemSpider | 54772 |
| DrugBank | DB08794 |
| ECHA InfoCard | ECHA InfoCard: 100.003.265 |
| EC Number | 203-761-9 |
| Gmelin Reference | 7551 |
| KEGG | C08261 |
| MeSH | D008010 |
| PubChem CID | 8042 |
| RTECS number | WS7875000 |
| UNII | 3T8B169ZQ1 |
| UN number | UN3265 |
| Properties | |
| Chemical formula | C8H14O4 |
| Molar mass | 174.19 g/mol |
| Appearance | Colorless liquid |
| Odor | Fruity |
| Density | 1.071 g/mL at 25 °C (lit.) |
| Solubility in water | Slightly soluble |
| log P | 0.78 |
| Vapor pressure | 0.03 mmHg (20 °C) |
| Acidity (pKa) | 10.7 |
| Basicity (pKb) | Diethyl Succinate has a pKb of approximately 24 (very weak base) |
| Magnetic susceptibility (χ) | -47.5e-6 cm³/mol |
| Refractive index (nD) | 1.419 |
| Viscosity | 1.887 cP (25°C) |
| Dipole moment | 2.84 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 298.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -743.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2413.2 kJ/mol |
| Pharmacology | |
| ATC code | N02BG10 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02 GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Precautionary statements for Diethyl Succinate: "P261, P264, P271, P272, P280, P302+P352, P312, P321, P362+P364, P501 |
| Flash point | Flash point: 117 °C |
| Autoignition temperature | 415 °C |
| Lethal dose or concentration | LD50 (oral, rat): 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 6800 mg/kg |
| NIOSH | WN4725000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Diethyl Succinate is not specifically established by OSHA. |
| REL (Recommended) | 5 g |
| Related compounds | |
| Related compounds |
Diethyl malonate
Diethyl adipate Diethyl oxalate Dimethyl succinate Succinic acid Diethyl fumarate |