
Diethyl glutarate carries a story that traces back to both war-time industrial needs and the rise of modern organic chemistry. During the twentieth century, chemists looked for ways to bring efficiency and scalability to the synthesis of esters for use in manufacturing and pharmaceuticals. Practically, diethyl glutarate found its feet as chemists searched for five-carbon chain molecules with reactive points suitable for polymer development. The molecule moved from a curiosity in early esterification studies to a staple in labs by the 1960s, riding the wave of expanding organic synthesis methodology. As polyesters and flexible plastics became essential, so grew the need for glutaric acid and its esters.
Glancing at clear liquid in a bottle or drum, diethyl glutarate gives little away about its impact. Chemists value its low volatility, stability, and miscibility with common organic solvents. It enters blends and transformations with ease, acting as both a reactant and a medium. Speciality manufacturers turn to it for intermediate steps, especially when branching out from simple diesters into more functionalized products. Laboratories hold it on their shelves for ester hydrolysis demonstrations, alkylation studies, and the pursuit of targeted molecular architectures. Its price point and availability slot it nicely into both academic and large-scale settings.
Diethyl glutarate has a molecular formula of C9H16O4 and a molecular weight of about 188.22 g/mol. In the bottle, it appears as a colorless to pale yellow liquid, usually free of particulate. Boiling point hovers around 215°C and it sports a mild, slightly fruity scent that sometimes reminds older chemists of early years in teaching labs. Density sits near 1.018 g/cm³ at 20°C. It resists reaction with water at room temperature, but its ester bonds break under basic or acidic conditions. The structure features an unbranched five-carbon skeleton with two ethyl ester groups—a combination that provides both flexibility for reaction and enough stability for storage. Flammability isn’t a primary concern but heating generates fumes that need proper ventilation.
Quality-focused suppliers grade diethyl glutarate by minimum purity, commonly 98% or higher. Key reference values include acid value, water content, and color (APHA scale). The typical label includes an identifier such as “Diethyl Pentanedioate” or designated product code, alongside hazard pictograms warning of potential eye and skin irritation. Storage advice focuses on keeping the container sealed in a cool, dry spot—moisture speeds up hydrolysis, turning some of the product into glutaric acid. For those shipping drums across state lines, compliance with transport regulations sits right alongside accurate labeling.
A well-trodden laboratory route prepares diethyl glutarate from glutaric acid and ethanol in the presence of a strong acid catalyst, often sulfuric acid. Glutaric acid dissolves in excess ethanol and the reaction mixture heats under reflux conditions—water condensing out and moving into a trap as the reaction proceeds. After several hours, the crude mixture cools, neutralizes, and goes through a separation to remove excess ethanol and byproducts. Distillation under reduced pressure gives a pure ester, suitable for most synthetic needs. In industrial settings, this esterification scales with efficient heat recovery and byproduct recycling, pushing yields and minimizing waste streams.
The two ethyl esters on diethyl glutarate invite all kinds of chemical transformations. Saponification opens the molecule into glutaric acid as both a teaching point and a route to other products. Reduction with lithium aluminum hydride cuts the esters straight down to 1,5-pentanediol, key for polyesters and certain pharmaceutical intermediates. Nucleophilic substitution tricks open one or both esters, while carefully controlled transesterification sometimes swaps out the ethyl groups for more exotic alcohols. Diethyl glutarate’s flexibility isn’t just academic; it allows manufacturers to tailor production lines for custom intermediates without changing their fundamental feedstocks.
Chemists and suppliers refer to diethyl glutarate with a handful of synonyms: Diethyl pentanedioate, Glutaric acid diethyl ester, and Pentanedioic acid, diethyl ester pop up most often on CAS registries. In marketing literature or safety data sheets, it might appear as DEGA or under proprietary blend trade names from chemical companies. No matter the alias, the substance behind the label traces back to the same five-carbon backbone and two ester groups.
People working with diethyl glutarate need basic chemical hygiene. Lab techs always wear nitrile gloves and goggles because skin or eye contact brings the familiar sting of esters—something most research veterans recall from spills during undergraduate practicals. Inhalation rarely presents a problem at room temperature, but warming a reaction vessel speeds up vapor, so local exhaust and fume hoods become important. Documentation from the European Chemicals Agency points out possible irritation and recommends swift rinsing and medical attention upon significant exposure. Storage remains straightforward: stable at standard room conditions, separated from strong acids or bases except during preparation steps. Waste protocols demand collection in flammable organic streams rather than down municipal drains.
Diethyl glutarate finds its real-world value serving as an intermediate in organic synthesis, especially for specialty plastics, resins, and certain active pharmaceutical ingredients. Its use as a flavoring agent or fragrance base owes more to its volatility and mild scent than to regulatory approval—here, other esters dominate consumer use. Manufacturers seeking biodegradable polyesters often look toward glutarate-based chains to build flexibility and toughness without introducing unwanted aromatic content or persistent contaminants. In some agrochemical syntheses, diethyl glutarate forms the backbone for pesticides and growth regulators. Skills picked up in graduate school with this ester carry over smoothly to troubleshooting scalable reactions on pilot plant floors.
Innovation with diethyl glutarate continues to surface, mostly driven by green chemistry and the search for biobased feedstocks. Researchers examine catalytic processes to lower energy inputs for esterification steps, avoiding strong mineral acids and acidic waste. Enzymatic synthesis, using esterases from microbial sources, opens doors to milder reactions with less environmental impact—a trend gaining steam with universities and specialty chemical firms. Some teams experiment with functionalizing the ester in single-step processes, aiming to reduce waste in pharmaceutical intermediate preparation. These advances focus as much on sustainability as cost, as environmental regulations grow stricter year after year.
Toxicology reports on diethyl glutarate are mostly reassuring at moderate doses, but safety testing remains crucial for any chemical in broad use. Researchers found limited acute oral and dermal toxicity, but chronic exposure brings risks of irritation to skin and mucous membranes. Most occupational exposures center on handling large volumes during esterification or distillation; personal history tells me ventilation systems and regular glove changes reduce risks to near background levels in typical labs. Moving into animal testing, no clear evidence of long-term carcinogenicity surfaced—though regulatory bodies always push for more data and safer substitutes. Wastewater treatment teams keep a watchful eye on ester breakdown products in effluent, aiming to prevent unforeseen ecological effects.
Diethyl glutarate stands at a crossroads shaped by sustainability goals and the continual pressure for cost-effective synthesis. The push towards renewable sources means that future supplies may increasingly come from fermenting plant-derived glutaric acid, displacing petroleum. Demand for safer, less toxic intermediates will likely boost research into alternative catalysts, solvent-free processes, and closed-loop recycling in major production sites. Polyesters made with glutarate esters could carve out a niche as more governments regulate single-use plastics. Synthetic chemists may look to embed reactive handles on diethyl glutarate, opening the doors to multifunctional biomaterials. In my own experience, students find early success using this ester as both teaching tool and research bridge—pointing to a future where practical, safe molecules enable bigger leaps in green chemistry.
Across industrial labs and chemical production floors, diethyl glutarate plays a bigger role than people imagine. This colorless, nearly odorless liquid may not grab public attention, but it shapes products we rely on more than folks realize. While working in a lab that specialized in organic synthesis, I saw firsthand how diethyl glutarate smoothed processes for both researchers and factory lines.
Chemists love tools that make molecule-building easier. Diethyl glutarate acts as a building block, helping create plastics, pharmaceuticals, and fine chemicals. It’s a popular ester for making other specialty molecules. Being able to introduce glutaric acid residues into a project gives flexibility and opens doors for synthesizing new compounds dedicated to drug discovery, flavor agents, or fragrances.
Take pharmaceuticals as an example. Drug makers rely on starting materials with reactive groups in the right spots. With diethyl glutarate, their chemists modify it to fit precursor compounds for active pharmaceutical ingredients. I’ve watched drug development teams turn simple esters into valuable molecules by linking, clipping, and rearranging diethyl glutarate in creative chemical reactions. Success in this area streamlines production and sometimes even lowers medication costs.
Our dependency on plastics wouldn’t be possible without reliable intermediates. Manufacturers use diethyl glutarate to engineer specialty polyesters. By blending this ester in their process, they tailor the properties of polymers for applications tough enough to withstand real-world conditions—say, a car’s dashboard, food-packaging films, or custom adhesives. These tweaks in plastic formulas can make products more durable or flexible, giving companies a real competitive edge.
Eco-friendly chemistry has gained ground, and diethyl glutarate joins the conversation here as well. Scientists in green chemistry circles have included this compound to build biodegradable plastics. These alternatives break down more easily after use compared to older plastics. In college research projects, I saw grad students choosing diethyl glutarate over more stubborn chemicals to help create materials that won’t clog up landfills as quickly.
Some jobs need the right liquid environment to help complicated reactions along. Diethyl glutarate dissolves a wide range of organic substances. Whether it’s dissolving dyes for ink or coaxing stubborn polymers to mix evenly, this ester solves practical problems quietly in the background. Its relatively low toxicity makes it easier to handle than harsher industrial solvents, a fact that gave me peace of mind during longer lab shifts.
Like any chemical with industrial value, safety needs constant attention. Skin contact and inhalation risks come with diethyl glutarate, especially where bulk handling takes place. In my experience, gloves, goggles, and good ventilation kept incidents rare. Still, more industries should review their workspaces or invest in better personal protective equipment. Regulatory agencies publish exposure limits, but reinforcing a safety-first work culture matters just as much in daily routines.
Diethyl glutarate stands as a dependable workhorse in chemistry. While not many recognize its name, people feel its impact in health, convenience, and progress. Thoughtful use paired with respect for safety keeps this ester working for us, not against us.
It looks like a clear liquid. It smells a bit sweet. Diethyl glutarate gets used in making plastic, flavors, perfumes, and in some labs as a solvent. Its name may not show up in headlines, but it does show up in a surprising set of industries that make stuff we use every day.
To figure out if diethyl glutarate is toxic, it helps to check what scientists and regulators like the European Chemicals Agency, EPA, and the NIH say. Reports suggest that inhaling its vapor or letting it touch skin can cause irritation, but it rarely leads to severe problems in most folks with usual chemical exposure in a workplace. Still, nobody wants to end up with skin burns, so gloves and eye protection are smart. I’ve spent plenty of time in university labs and can confirm the “better safe than sorry” attitude is more than just advice.
Tests on animals show some effects at very high doses, especially with repeated exposure: things like weight loss, changes in liver cells, or upset breathing. No evidence ranks diethyl glutarate as a cancer risk, reproductive hazard, or major poison for humans at normal levels. Agencies rate it as having low to moderate acute toxicity. Compare it to more notorious chemicals—benzene, for example—diethyl glutarate just doesn’t land on the same danger list.
I asked two friends in chemical manufacturing about it. Both shrugged and said, “It’s handled with the same basic care as any solvent. No one wants to drink it, but there’s no mad rush to phase it out.”
What happens once it leaves a factory? This matters for those of us who care about clean water and soil. Luckily, diethyl glutarate breaks down in the environment faster than chemicals designed to last. Bacteria and sunlight do their job, and this compound does not stick around for years. It doesn’t accumulate in fish, and current research does not link it to big eco disasters.
Still, any spill or large dumping should be avoided. A big release could cause local harm, much like dumping out a tub of gasoline or paint thinner.
Many folks in chemistry spend their days around liquids like this. The best advice comes down to basic habits: wear gloves, use goggles, watch the ventilation, and don’t huff fumes indoors. The chemical industry has trained workers to prevent spills and cut off exposure routes. It works for this compound as well as most lab and manufacturing solvents.
For families living near plants, government agencies check air and water levels. Most states in the US require regular reports on volatile organic compounds. As of now, you’re much more likely to run into car exhaust or cigarette smoke as a health risk than to run into a dangerous dose of diethyl glutarate.
Regulators can step up by keeping monitoring rules tight and encouraging research into long-term low-level effects. Educators ought to include real-world chemical safety training in every technical course. If companies keep up with responsible handling and public transparency, most problems stay small.
Diethyl glutarate deserves attention, but with respect more than fear. Education, strong workplace habits, and good regulations do more to limit toxic harm than shock headlines ever will.
Digging into the chemical side of things, Diethyl Glutarate takes on the formula C9H16O4. Picture a molecule made from nine carbons, sixteen hydrogens, and four oxygens. On paper, that looks simple. Out in the world, this molecule shows up in research labs, specialty manufacturing, and sometimes even fragrance compounds.
When jobs call for glutaric acid esters like Diethyl Glutarate, it’s because the structure gives it unique qualities. It doesn’t just serve as another chemical; it solves specific needs, especially in organic synthesis, coatings, and solvent roles. A handful of carbons strung in a five-carbon chain, flanked by two ethyl ester groups, opens up its flexibility. People interested in eco-friendly solvents or specialty synthesis see this molecule as a cleaner option compared to more volatile choices.
Pulling from trusted research and regulatory data is key when talking chemistry. Diethyl Glutarate pops up on chemical safety lists, with the National Center for Biotechnology Information offering plenty of detail. Chemists in industry don’t just memorize formulas; they care about exposure, handling, and disposal. The US EPA and European Chemicals Agency provide breakdowns of potential risks, so teams can figure out smart ways to use the material safely. It’s dense, but the chemical formula tells you about reactivity, possible side effects, and storage rules. That’s where experience with chemicals comes in handy. Some people overlook how a simple ester group turns the handling game, with less odor and toxicity than raw acids.
Living with chemicals in the lab or plant means paying attention to the details. Diethyl Glutarate runs low on toxicity compared to many of its siblings, but skin contact and inhalation might bring irritation. It’s not the sort of risk you want to ignore. Responsible use demands personal protective equipment and a ventilation plan. Disposal shouldn’t just mean dumping it down the drain, either. Local regulators have strict guidelines for organic waste, especially solutions or residues. People following protocols and referencing up-to-date Material Safety Data Sheets keep things safe for workers and the environment.
Safer practices call for clear labeling and comprehensive training. Teams who work with esters like Diethyl Glutarate take the time to review lab notes, practice emergency response, and monitor storage conditions. Labs and plants embracing green chemistry pick Diethyl Glutarate as a step toward better air quality and fewer hazardous emissions. Swapping sharp-smelling, harsh chemicals for less aggressive ones can improve both worker morale and long-term health. That’s not just good policy—it’s smart stewardship.
Thinking back to early projects in a chemistry lab, confusion often started with not fully grasping the structural formula. Once you understand what C9H16O4 means, planning reactions and identifying safe working limits gets easier. Teams relying on correct formulas avoid mix-ups in multi-step syntheses. That’s not just about chemistry; it’s about efficiency and reliability. Chemical literacy starts with basics like molecular formula and builds into safe, successful projects.
Diethyl glutarate isn’t a chemical that most folks run across every day, but for those working in labs or certain manufacturing settings, it plays a bigger role than most realize. The stuff comes with its own risks and quirks — the kind that don’t just go away with a casual cap on a bottle. Over the years, I’ve seen what can happen if someone treats storage like an afterthought. A leak here, an odd smell there, and suddenly a whole workbench or storeroom gets cleared out because fumes became an issue.
Everyone likes to talk about safety data sheets, and for good reason. They spell out the basics: diethyl glutarate prefers cool, dry places far from sources of heat. Leave it near a sunny window or a radiator, and the risk of evaporation or a pressure build-up rises. Most labs take the advice to heart, stashing bottles in temperature-controlled cabinets or tucked at the back of a cool storage room. I’ve seen what happens when corners get cut — warping caps, discolored liquids, even that distinctive sharp odor escaping long before clerks notice.
Air is the enemy of many chemicals, and diethyl glutarate fits that bill. Air and moisture can slowly mess with the stuff in ways that might not show up until months down the road. A tightly shut container with a well-fitted cap beats the temptation to “just snug it down” any day. Glass bottles with solid screw tops or fluoropolymer-lined caps see a lot of use for a reason. I’ve learned to double check lids before putting anything back on the shelf. That simple step saves a lot of headaches later.
Mislabeling or sloppy record-keeping turns a routine storage task into a problem. More than one lab has lost expensive stock because someone grabbed an unlabeled jar thinking it was something else. I write clear dates and initials on every fresh container, and anyone working with me does the same. Computerized inventory logs help, but nothing beats an old-fashioned label. The extra minute spent here saves plenty of confusion, especially during safety audits.
Some folks never realize that even a clean-looking chemical cabinet can hide risks. Mixing diethyl glutarate with oxidizers, strong acids or bases, or storing it next to reactive metals opens up a whole world of trouble. I’ve worked in spaces where folks separated shelves by hazard type, keeping “friends and foes” apart. Spills still happen, but keeping like-with-like goes a long way to limiting bad reactions.
Even with the best laid plans, accidents happen. Absorbent pads, proper ventilation, and PPE like gloves and goggles are regular features wherever chemicals are used. I can remember one near-miss where a quick mop-up with a proper spill kit kept things safe for everyone around. Not a week goes by without someone running a drill or refresher for what to do if things go sideways.
Safe storage starts with the right shelf and the right cap, but it carries on through daily habits, labels, and training. Respect the risks and show the same respect for safety procedures, and diethyl glutarate doesn’t have to be any scarier than a locked toolbox. I’ve found the real trick is making safety a shared responsibility. That creates a space where people look out for one another, and the chemical stays in its place until needed — and no one gets caught off guard.
Holding a clear glass bottle of diethyl glutarate in the lab, the liquid almost fools you into thinking it’s just water. It pours easily, has a faint fruity odor, and nowhere near the volatility of something like ether. This ester runs as a colorless, slightly oily substance, moving with enough fluidity to fill up a beaker fast. Density for this compound sits around 1.02 grams per cubic centimeter, putting it right in the ballpark of water but heavier than oil. This makes handling and transferring it straightforward—there’s none of that wild splash you see with more volatile solvents.
Diethyl glutarate boils at about 218 degrees Celsius. That may not seem outlandish, but in an organic chemistry room, reaching that temperature means you’re pretty far up the hot plate dial. On the other hand, it solidifies just below -70 degrees Celsius. It takes seriously cold conditions to freeze this liquid up into a solid block. This wide liquid range is what helps manufacturers use it in some specialty syntheses and as a solvent when ordinary alcohols don’t cut it.
I remember testing diethyl glutarate’s solubility back in a university project—this stuff doesn’t play nicely with water. Instead, it prefers to hang out with organic solvents. You can mix it easily with compounds like ether, acetone, and even chloroform. Water shakes up with it for a bit, but doesn’t fully combine. That trait makes it valuable in situations where you need to separate organic mixtures from water, whether you’re cleaning up a reaction or extracting flavors in industry.
Vapor pressure plays into safety a lot in any lab. With diethyl glutarate, the vapor pressure is low—just about 0.2 mm Hg at room temperature. I’ve kept open containers on the bench for short periods without losing too much to the air. Compared to high-evaporation solvents, it feels pretty forgiving in terms of exposure risk. Still, the fruity, ester-like scent can build up if ventilation isn’t great. It’s worth sticking to fume hoods because irritation or long-term exposure can’t be written off.
From pharmaceuticals to flavors, diethyl glutarate’s physical qualities are why chemists reach for it. The manageable density, stubborn resistance to freezing, and friendly relationship with organic solvents set it apart. In synthesis or extractions, those characteristics cut work time and waste. I’ve seen it used as a reaction medium for making plasticizers—both for its chemical characteristics and because it maintains stability at higher reaction temperatures.
Safe handling always connects back to understanding these physical properties. Training should stress that even low-volatility liquids like diethyl glutarate demand respect. Good gloves and goggles keep skin protected, and proper waste disposal avoids environmental hazards. It’s a solid example of a tool that, in the right hands, gets the job done safely and efficiently.
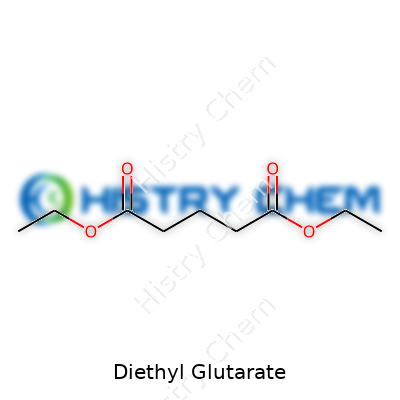
| Names | |
| Preferred IUPAC name | Diethyl pentanedioate |
| Other names |
Glutaric acid diethyl ester
Diethyl pentanedioate Pentanedioic acid, diethyl ester Diethyl 1,3-propanedicarboxylate |
| Pronunciation | /daɪˈɛθ.ɪl ˈɡluː.tə.reɪt/ |
| Identifiers | |
| CAS Number | 701-55-9 |
| Beilstein Reference | 635293 |
| ChEBI | CHEBI:88543 |
| ChEMBL | CHEMBL510872 |
| ChemSpider | 15691 |
| DrugBank | DB14636 |
| ECHA InfoCard | 100.006.722 |
| EC Number | 203-743-1 |
| Gmelin Reference | 8097 |
| KEGG | C14037 |
| MeSH | D002950 |
| PubChem CID | 8058 |
| RTECS number | MA4375000 |
| UNII | 9W6K7Q9Z5K |
| UN number | UN2367 |
| Properties | |
| Chemical formula | C9H16O4 |
| Molar mass | 218.28 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Mild odor |
| Density | 1.023 g/mL at 25 °C |
| Solubility in water | slightly soluble |
| log P | 0.91 |
| Vapor pressure | 0.03 mmHg (20°C) |
| Acidity (pKa) | 25.6 |
| Basicity (pKb) | Diethyl Glutarate has a pKb of 24.4 |
| Magnetic susceptibility (χ) | -6.46e-6 cm^3/mol |
| Refractive index (nD) | 1.417 |
| Viscosity | 2.37 mPa·s (25 °C) |
| Dipole moment | 4.57 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 527.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -743.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3940.8 kJ/mol |
| Pharmacology | |
| ATC code | V09IX10 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P337+P313, P370+P378 |
| NFPA 704 (fire diamond) | 1-2-0-0 |
| Flash point | 117 °C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD50 oral rat 8191 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 6916 mg/kg |
| NIOSH | WWG8530000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 mg/m³ |
| Related compounds | |
| Related compounds |
Glutaric acid
Glutaronitrile 1,5-Pentanediol Dimethyl glutarate Diisopropyl glutarate Di-n-butyl glutarate |