
Some molecules carry a quiet legacy in the world of chemistry, and cyclopentanemethanol counts as one of those. Early synthetic organic chemistry in the mid-twentieth century saw researchers attempting to modify simple ring systems, and the five-membered ring scaffold of cyclopentane stood out as a jumping-off point. Scientists searching for new building blocks noticed that adding a single methanol group to cyclopentane opened doors for more functional compounds. Over the decades, investments in petrochemical research led to methods that offered higher yield and fewer by-products. Laboratories shifted from batch syntheses using strong acids and high pressures to healthier, greener techniques as environmental awareness grew. The historical growth of cyclopentanemethanol production reflects a broader shift in chemical manufacturing methodology—balancing industrial utility with safety and sustainability concerns.
Cyclopentanemethanol isn’t something that shows up on a grocery shelf or in a typical repair shop. In my laboratory experience, it mostly finds its way into glass bottles labeled for specialty synthesis purposes. Chemists see it as a useful tool—a nonaromatic alcohol with a compact, rigid structure that can serve as a backbone for more elaborate molecular designs. The hydroxymethyl group offers a handle for attaching new groups or creating linkages used in pharmaceuticals, fragrances, and sometimes specialty plastics. For practical handlers, that means the chemical wears many hats across several sectors—its quiet versatility earns it a spot on the shelf of many research benches.
On the bench, cyclopentanemethanol looks pretty unassuming: it presents as a colorless liquid, sometimes with a faint odor reminiscent of rubbing alcohols. The boiling point clocks in just above 180°C, which gives it reasonable thermal stability for most synthetic operations. It comes with a density close to water, and its solubility profile strikes a nifty balance: enough to dissolve in common organic solvents, reluctant to blend with water in large amounts. The hydroxyl function makes it moderately reactive, but the five-membered ring slows down unwanted side reactions, so it stores well if sealed properly. When I’ve worked with it, gloves and goggles are standard—its vapors don’t irritate much, but it’s best not to test that comfort.
Labels for cyclopentanemethanol usually call out purity above 97%, especially for applications in analytical chemistry or API intermediates. Suppliers include batch numbers, CAS identification, hazardous material pictograms, and storage precautions. Researchers and manufacturers scan for details like water content, GC assay, and typical impurity profiles. Some lots come with spectroscopic data, like NMR or IR, which is especially useful for academic projects or drug discovery workflows. I've seen more suppliers jumping on QR-coded safety datasheets right on the label—a practical step for faster compliance.
Producers reach for several synthethic routes depending on the facilities and downstream plans. One common approach involves reduction of cyclopentanecarboxylic acid or its derivatives, typically using lithium aluminum hydride or sodium borohydride. Some methods use catalytic hydrogenation under a flow of hydrogen gas. In modern labs concerned about waste and energy, enzymatic or catalytic transfer hydrogenation sees increased attention. As green chemistry pushes forward, more scalable setups skip harsh metals or extreme conditions. My own work used a cautious approach—avoid overheating the flask, keep moisture out, and the yield stays high.
Cyclopentanemethanol acts as a flexible platform for organic synthesis. The primary alcohol group means esterification comes easily—combine with carboxylic acids and a little acid catalyst, and the result gives a cyclopentyl ester. Oxidation under the right conditions—think PCC or mild chromates—produces cyclopentanecarboxaldehyde, which opens further routes in complex molecule construction. Conversion to halides through substitution techniques gives chemists new handles for further modification. In drug synthesis, the rigid cyclopentyl ring often serves as part of active pharmaceutical ingredient structures, imparting metabolic stability or unique pharmacokinetic properties.
Cyclopentanemethanol turns up under a few labels depending on suppliers and databases: cyclopentylmethanol, (hydroxymethyl)cyclopentane, or CPM alcohol. CAS number 1678-47-5 serves as the global chemical language for procurement and inventory. I’ve seen it listed with subtly different trade names—rarely flashy, usually technical—signaling its chief market: research and specialist manufacturing.
Direct handling demands care typical for mid-weight organic solvents—ventilation, nitrile gloves, and eye protection. Cyclopentanemethanol doesn’t present catastrophic hazards at lab scale, yet vapors should not be inhaled, and repeated skin exposure can dry or irritate. Laboratories train staff to keep open bottles capped, and waste collection happens in flame-rated containers: even low-volatility alcohols pose fire risks near heat sources. In industrial settings, process safety walks hand-in-hand with emission controls and real-time spill monitoring. Storage stays simple—cool, dry, away from acids and oxidizers. For transport, UN-numbered hazardous goods protocols keep things predictable and compliant.
Pharmaceutical research leans heavily on cyclopentanemethanol as a key intermediate, especially for certain antiviral and cardiovascular drug families. Its rigid structure can mimic biological motifs, helping medicinal chemists fine-tune how a drug behaves. Outside pharma, plasticizers, high-performance polymers, and specialty coatings sometimes draw on its unique effects. Perfume formulators use it for musky, woody top notes that don’t evaporate as fast as lighter alcohols. In my time consulting for fragrance startups, I saw how niche synthetic alcohols shape long-lasting scent profiles without overpowering the main composition. Its use in agricultural chemistry and electronics remains a specialist endeavor, giving it a resilience against sudden shifts in market demand.
Academic labs and major industrial R&D groups keep cyclopentanemethanol in rotation for exploring new reaction mechanisms and synthesizing model compounds. Method development for greener synthesis gains traction, using recycled catalysts and energy-efficient setups. Biomedical research investigates its derivatives for antiviral and neuroactive properties, given the ring’s structural similarity to several endogenous molecules. Intellectual property filings keep rising as organizations jockey for new cyclopentyl-derived drugs and specialty materials. From my own perspective, securing sustainable supply chains for such narrow-use chemicals means joining industry consortia and fostering supplier transparency—a strategy that’s paid dividends in both cost and reliability.
Published toxicity data show moderate acute oral toxicity in lab animals, with typical LD50 values lying in the gram-per-kilogram range. No strong evidence for carcinogenicity or long-term harm has emerged, yet chronic exposure lacks exhaustive investigation. Cytochrome P450 studies indicate that the compound undergoes oxidation chiefly at the methyl alcohol group, resulting in predictable metabolites. Worker safety organizations recommend limiting airborne concentrations and ensuring prompt decontamination of spills. In my experience, prudent lab hygiene—frequent glove changes, proper fume extraction—keeps risks squarely in check.
The future for cyclopentanemethanol seems stable and quietly promising. Demand won’t rocket to the headlines, but ongoing discoveries in medicinal chemistry and advanced polymers point toward niche growth. Process intensification, greener catalytic systems, and digital supply chain management look set to increase access while holding down environmental impact. Education and workforce training in handling specialty chemicals maintain an edge for labs and manufacturers. If regulatory landscapes stay supportive, cyclopentanemethanol will remain a valued tool for those working at the cutting edge of synthesis and design, proving there’s lasting value in compounds that may not grab mainstream attention but continue to open new possibilities across science and industry.
Cyclopentanemethanol often lands on supply lists for research chemists and factory workers alike, but it rarely gets a spotlight outside those circles. Its name sounds intimidating until you take it apart. This chemical, made up of a five-membered carbon ring with a small alcohol group attached, has properties that tuck it nicely into several corners of modern industry.
Drug makers appreciate Cyclopentanemethanol for more than just its structure. They rely on it while building bigger, more complicated molecules, using it as a starting point or a building block. I’ve watched colleagues in the lab reach for it during processes that demand steady reactivity and a bit of flexibility. Researchers harness its ring structure to sneak rigidity into compounds that need to stay put in biological systems. Some antiviral and anti-inflammatory compounds take shape through routes that involve this molecule in early steps.
Walk through a large fragrance manufacturer, and you’ll notice tanks with odd labels. Cyclopentanemethanol sits among them because it offers a gentle push in creating aroma chemicals. It doesn’t smell like much on its own. Still, run it through a few chemical transformations and it shapes backbone ingredients for perfumes and flavorings. At scale, costs matter, and this alcohol balances price with performance. Producers know that a tweak in starting materials sends ripples through scent and taste profiles, so choosing solid base chemicals like Cyclopentanemethanol matters to their business.
Plastic engineers sometimes blend Cyclopentanemethanol into specialty polymer formulas. Its physical footprint—neither too bulky nor too simple—lets it modify melting points and mechanical strength. I recall a polymer chemist explaining how even small changes in an alcohol group can push a polymer from brittle to tough. This chemical’s ring helps resist breakdown too, which appeals to those designing gear for tough environments.
Like any industrial chemical, handling Cyclopentanemethanol brings safety concerns. It won’t explode on contact or corrode wrappings, but standard gloves and goggles keep things safe. Most suppliers do a good job tracking purity, and reputable sources help prevent mix-ups that risk both health and final product quality. Labs and factories both benefit from extra attention to detail here—small errors can domino into big problems, especially in food or pharma.
Plenty of debate rumbles on about greener chemistry. Cyclopentanemethanol shares those challenges. Its main route today starts with fossil fuel derivatives. Researchers want cleaner methods, so green labs chase plant-based feedstocks and smarter catalysts. Lowering waste and boosting efficiency will push costs lower, open doors for new products, and limit environmental load. This isn’t pie-in-the-sky talk—improvements often start with one small chemical used in a dozen places. Every percentage point of efficiency counts when a factory ships by the barrel.
Cyclopentanemethanol holds its ground in several industries not because it’s flashy, but because it does its job well. Small changes in base ingredients can transform entire markets, from medicine to fragrance. If we keep a steady focus on safer practices and smarter sourcing, we’ll get the best from chemicals like this without giving up on responsibility.
Chemistry shapes daily life, from plastics to medicines. Cyclopentanemethanol is one of those compounds that sometimes pops up in labs, especially in organic synthesis. Its chemical formula reads as C6H12O, marking it as a six-carbon compound with twelve hydrogens and a single oxygen. It carries a cyclopentane ring structure—a five-membered carbon ring—anchored to a single methanol group (–CH2OH).
Many folks hear chemical names and tune out, thinking they're only for experts. The truth is, a compound like cyclopentanemethanol carries more real-world weight than its name suggests. In organic chemistry, ring structures like cyclopentane change the chemistry of a molecule, often lending it stability or unique reactivity. The attached methanol group makes it capable of forming hydrogen bonds, opening doors for further reactions. In practice, chemists use it as a building block, a way to tweak more complex molecules for pharmaceuticals and specialty materials.
If you walk into a college lab or a research plant, you can spot cyclopentanemethanol in use for making other organic molecules. That alcohol group (the OH) means it interacts well with acids and can join up with other chemicals, such as in esterification or oxidation reactions. Personally, I remember setting up reactions that used cyclopentanemethanol to synthesize intermediates for bioactive compounds. Every carbon and hydrogen in the formula controls how it reacts with other materials, whether that means holding its ring structure steady during a tough reaction or donating its alcohol group to shift the balance of molecules in a flask.
Understanding a formula goes beyond memorizing a string of letters and numbers. Chemists rely on knowing the atomic makeup to predict risks, behavior, and usefulness. The formula C6H12O tells us the compound is relatively non-volatile compared to lower-weight alcohols like methanol or ethanol, and the single oxygen means it reacts as an alcohol without displaying acidic behaviors. Scientists who formulate drugs or design materials lean on these details for safety and efficiency. A small change in formula flips the script—from something usable to something hazardous, or from a useful intermediate to an unwanted byproduct.
While working with cyclopentanemethanol, safety isn’t just a checklist. Its ring structure means it's not expected to rapidly break down in the environment. So, safe disposal matters, reducing the release into waterways or soil. Training in responsible handling pays off, not only for the chemist at the bench, but for the broader community. Chemists can switch to greener solvents and explore pathways that minimize hazardous byproducts—this kind of step-by-step improvement, rooted in formula knowledge, builds safer labs and healthier surroundings.
Understanding cyclopentanemethanol isn't only for academics or specialists. The chemical formula, C6H12O, underpins how the molecule acts, how it can be handled safely, and how it fits into bigger picture processes. The story behind a compound’s formula reveals far more about science’s place in daily life than a list of letters and numbers. Knowing where and how to use something like cyclopentanemethanol sets apart careful, responsible industry from reckless practice.
Cyclopentanemethanol often shows up in laboratories, chemical factories, and research institutions as a colorless to pale liquid. Folks working in organic synthesis and pharmaceutical development might know it as a handy building block. Seeing an unfamiliar chemical name sparks the same questions as any odd substance on a shelf: Is it dangerous? Does it come with health risks?
The biggest concern in any workplace handling chemicals comes down to health. No widespread reports link cyclopentanemethanol to acute toxicity in humans, based on available data. The United States Environmental Protection Agency doesn’t list it as a carcinogen or notorious poison. Animal studies, some seeking to clarify its impact, point to low acute toxicity. Researchers administered moderate-to-large doses to rodents and observed few symptoms beyond mild irritation or discomfort, with rare fatality.
Experience matters most in the real world. Handling this compound without gloves left my hands feeling oddly dry and irritated. No burning, no emergency, but a few hours of skin protest. Reading more, I learned that direct skin or eye contact could result in local irritation. It doesn't attack organs or the nervous system at low doses—unlike notorious solvents like toluene or methanol. Inhalation doesn't bring the sharp headaches or dizziness that some chemical vapors cause, although nobody should intentionally breathe in lab vapors.
Cyclopentanemethanol doesn't linger in soil or water as long as other industrial solvents. According to studies from European chemical watchdogs, it biodegrades fairly quickly. No evidence links it with bioaccumulation in fish or crops. That's reassuring for workers and communities living near plants using this substance, but industrial releases never mean zero concern. Environmental damage often comes less from what a compound directly does, and more from what people do with waste.
Flammable liquid warnings matter. Cyclopentanemethanol won't set a beaker aflame like gasoline will, but it ignites around 87°C (189°F). Open flames or sparks turn a minor spill into an accident scene. Lab practice already demands flame-resistant coats and fire extinguishers nearby. In crowded workspaces, discipline starts slipping when folks get comfortable. I’ve seen minor accidents from solvents far less flammable, so I always remind colleagues that safe practice has to be muscle memory—not an afterthought.
Safe handling calls for gloves—nitrile and neoprene top the charts for chemical protection. Well-ventilated hoods keep vapors away from faces. Goggles prevent stinging splashes. Spill kits and absorbent pads should sit within arm’s reach. Emergency showers and eyewash stations save precious seconds. Proper labeling and storage keep accidents in check.
Disposal takes priority too. Cyclopentanemethanol waste stays in well-marked containers, never dumped down the drain. Sending waste to licensed chemical processors stops harmful substances from entering rivers and soil. Companies need regular safety training and audits. Practicing drills and reviewing safety data sheets get everyone on the same page ahead of emergencies.
No chemical, however benign by most standards, deserves casual treatment. Cyclopentanemethanol rarely makes headlines for causing harm, but complacency leads to trouble. Following common-sense safety rules and keeping up with the latest hazard data lets folks work safely while pushing research and development forward.
Cyclopentanemethanol isn’t a household name, yet in the lab or production line, this chemical plays a role in solvents, intermediates, and more. The boiling point—roughly 186 to 187 Celsius according to most reliable chemical catalogs—might look just like a number. But it tells us a lot about how this compound behaves. For those making batch processes run smoothly or ensuring safety protocols are followed, knowing this property can make or break a synthesis.
Every chemist I know has a story about a runaway reaction or a slow distillation. A boiling point isn’t just a textbook answer. Let’s say you’re purifying cyclopentanemethanol by distillation. Jumping above 186°C means vapors form fast and, without proper setup, you’re on your way to product loss or even an accident. At the same time, if you operate far below this temperature, purification drags on and resources get wasted. Those who work with glassware, heating mantles, or reactors pick up early that there isn’t a substitute for knowing these practical numbers.
Reliable references like the Sigma-Aldrich chemical catalog and the CRC Handbook record the boiling point of cyclopentanemethanol at 186–187°C under normal atmospheric pressure. Academic studies back this up—there’s solid agreement. This consistency matters. Researchers, engineers, and plant operators across the world can be confident they’re reading from the same playbook. Handling protocols depend on these properties. Improper boiling data leads to failed runs, waste, and in worst-case scenarios, unsafe working environments.
There’s also the question of emissions. At 186°C, uncollected vapor escapes into the air during open heating. This isn’t just a product loss problem; it also adds to chemical exposure risks. Many labs and industries use closed systems and condenser traps for cyclopentanemethanol, taking the boiling point into account to pick the right setup and avoid releasing volatile organics.
Working with cyclopentanemethanol in practice, you notice how temperature changes can impact everything. Exposing the chemical to conditions above its boiling point in loosely capped containers means evaporation takes over. Containers swell or leak, leading to unnecessary cleanup or loss. Smaller differences in boiling points between chemicals can complicate separation. Knowing this compound boils at 186–187°C narrows down choices for separation protocols and informs solvent selection.
I’ve seen labs ignore boiling points and pay the price in ruined reactions and wasted hours. Safety teams count on these numbers to guide ventilation, protective gear, and emergency plans. Engineers designing larger-scale syntheses weigh this data when choosing materials for piping and vessels. Heat resistance, thermal expansion, and compatible seals all hinge on accurate property data.
Focusing on numbers from recognized chemical safety sheets and reputable journals also meets the spirit of scientific trust. There’s no room for error in process development, and facts like a boiling point of 186–187°C for cyclopentanemethanol form the foundation for next steps. From students mixing small samples to professionals overseeing metric tons, this piece of information isn’t trivia. It sets the rhythm for work, safety, and innovation in chemical sciences.
Most people haven’t heard of cyclopentanemethanol, but for those in labs or working with specialty chemicals, handling it safely means a lot. I’ve worked in research environments where proper storage makes the difference between a routine day and a disaster. Cyclopentanemethanol brings its own quirks. Folks who’ve spent time with alcohol-based solvents know: a bottle left in the wrong place can knock out productivity or, worse, set off emergencies.
Cyclopentanemethanol comes with a flammable label for a reason. Its vapors catch a spark quicker than you might expect. It may not shout danger like some corrosives, though it doesn’t forgive neglect. Locking it away from heat is more than a suggestion. No sunny windowsills or spots under heat lamps. I’ve seen someone stash a bottle on a high shelf near a radiator, thinking it safer off the beaten path — until that warmth turned a minor mistake into an emergency. Keep it cool, ideally below room temperature, in a well-ventilated flammables cabinet.
Shelving chemicals alphabetically seems easy, but storage calls for understanding what reacts with what. Cyclopentanemethanol belongs nowhere near strong oxidizers or acids. A spill could create toxic fumes or fire. I remember a senior tech hammering this point: check every label, every time. We sorted bottles into color-coded bins, not just for looks, but for separation. Simple steps like this prevent accidental chemical reactions if bottles break.
Moisture and cyclopentanemethanol don’t mix. Some chemical grades degrade when exposed to air, so tight seal matters. I’ve known colleagues to slap on parafilm or vacuum grease, whatever it takes to keep a crisp seal. Avoid decanting into larger containers. The less air in the bottle, the better. This cuts risks of contamination and keeps the contents effective for future experiments or synthesis.
Proper storage doesn’t stop at putting a bottle away. Good record-keeping stays just as important. I’ve seen accidents traced back to old or unlabeled containers. Label dates when each bottle opens, track amounts used, and check for any changes in color or smell that point to degradation. If you notice a problem, treat it seriously―flag it, contain it, and talk to trained staff before proceeding.
A fancy cabinet and fancy labels mean little if folks don’t respect protocols. Everyone handling chemicals should get hands-on training—and refreshers. In places where I’ve worked, the best safeguard wasn’t a set of rules; it was fostering habits through real practice. Review safety data sheets. Ask questions. Never shortcut steps, even late at night or when rushing a project.
Safe storage takes effort upfront, but it builds trust, efficiency, and peace of mind. If unsure about a step, reach out to experts or safety staff. Regulations and best practices keep shifting; staying current isn’t optional. Cyclopentanemethanol won’t grab headlines like more famous chemicals, but it still deserves respect—and vigilance—every day it’s on your shelf.
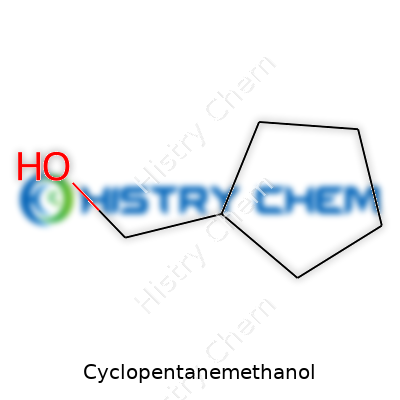
| Names | |
| Preferred IUPAC name | Cyclopentanecarbinol |
| Other names |
CPM
Cyclopentane methanol Cyclopentylcarbinol Cyclopentylmethanol Hydroxymethylcyclopentane |
| Pronunciation | /ˌsaɪ.kloʊˌpɛn.teɪ.nəˈmɛθ.ə.nɒl/ |
| Identifiers | |
| CAS Number | [1003-18-1] |
| Beilstein Reference | 1320630 |
| ChEBI | CHEBI:77583 |
| ChEMBL | CHEMBL16230 |
| ChemSpider | 16284 |
| DrugBank | DB02185 |
| ECHA InfoCard | 100.066.018 |
| EC Number | 201-040-3 |
| Gmelin Reference | 8786 |
| KEGG | C06427 |
| MeSH | D003462 |
| PubChem CID | 73558 |
| RTECS number | GV7875000 |
| UNII | K4J3181QME |
| UN number | UN2246 |
| CompTox Dashboard (EPA) | DTXSID2021429 |
| Properties | |
| Chemical formula | C6H12O |
| Molar mass | 100.16 g/mol |
| Appearance | Colorless liquid |
| Odor | mild odor |
| Density | 0.948 g/mL at 25 °C(lit.) |
| Solubility in water | miscible |
| log P | 0.8 |
| Vapor pressure | 0.56 mmHg (25 °C) |
| Acidity (pKa) | 15.7 |
| Basicity (pKb) | 15.09 |
| Magnetic susceptibility (χ) | -54.2·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.452 |
| Viscosity | 2.033 cP (25°C) |
| Dipole moment | 2.19 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 338.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -259.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3272.8 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | Flash point: 89 °C |
| Autoignition temperature | 260 °C |
| Explosive limits | Explosive limits: 1.1–9.5% |
| Lethal dose or concentration | LD50 oral rat 4830 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Cyclopentanemethanol: "3200 mg/kg (rat, oral) |
| NIOSH | CY8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/m³ |
| IDLH (Immediate danger) | No IDLH established |