
Butyrolactone first caught the attention of chemists around the early twentieth century. Its roots tie back to the growing interest in heterocyclic compounds, a time when organic chemistry started transforming into the powerhouse that would feed industrial and pharmaceutical revolutions. Research in those days often involved trial and error, glass columns, handwritten notes, and sometimes, risks few would dare to take today. As labs grew more sophisticated and regulatory frameworks followed, scientists dug deeper, learning that this small, colorless liquid could serve as a foundation for complex chemical syntheses. The early decades saw butyrolactone moving from academic curiosity to an agent federal agencies watched more closely.
Butyrolactone comes as a clear liquid, weighing just heavily enough to remind you it's more than water but not so dense as to feel threatening in the hand. Markets sell it under different product names, but the substance remains the same—a five-membered lactone ring, one oxygen tucked into the structure, modest, but quietly reactive. Chemical suppliers market it for research and industrial work, often in bottles marked with thick black text warning of restricted use. Legitimate demand spans from laboratory reagent shelves to workshops mixing solvents or specialty coatings. There's a push-pull here: regulation grows, but chemistry finds new ways to use this handy tool.
Pour a little butyrolactone into a vial, and you’ll see a colorless, slightly oily liquid, quickly mixing with water and organic solvents alike. Its boiling point sits around 204°C, and it slips easily into solution, thanks to strong polarity and a modest molecular weight just above 86g/mol. The characteristic faint odor says little about its punch in chemical reactions. Under a microscope, it’s the electronegative oxygen and constrained ring that help crack open other bonds; the chemistry is sharp, even if the liquid appears mild at first glance. Solubility and stability let it work at a range of temperatures and pressures, which explains its popularity among tinkerers and industrial chemists alike.
Buy a container from any reputable supplier, the label lists gamma-butyrolactone or GBL, sometimes with a purity north of 99.5%, and warnings around toxicity, exposure, and legal use. Specifications include boiling point, melting point, water content, and a chemical assay. Each of those numbers matters—a little residual water, and downstream chemistry won’t go as planned. Regulations have changed the way suppliers package and ship it, requiring tamper-evident seals and shipping documentation strict enough to impress border officials. Industry standards give guidance on fire protection and emergency handling, because this liquid can react unpredictably when exposed to strong bases or acids.
Manufacturers take a few different routes to make butyrolactone, but most rely on dehydrogenation of 1,4-butanediol. Copper or silver catalysts do the heavy lifting, coaxing out hydrogen and knitting the lactone ring shut. Chemical engineers watch carefully as reaction temperature and pressure balance efficiency with purity. Others try more niche methods, such as oxidation of tetrahydrofuran (THF), but this route suits specialty plants better than the big producers. Downstream purification uses distillation or solvent extraction. Each batch requires careful monitoring for byproducts and impurities—otherwise, entire lots turn useless for anything but waste.
Butyrolactone doesn’t rest easy. Under alkaline conditions, it flips open to form gamma-hydroxybutyric acid (GHB), a transformation that carries legal risks in many countries. In high-school organic chemistry, you might hydrolyze it with acid or base, using the ring to teach about equilibrium and reaction pathways. In the lab, chemists tweak the molecule to add functional groups, making everything from solvents to photoresist developers for semiconductor lithography. Its versatility in reactions, from transesterification to polymerization, lets manufacturers build specialty polymers and resins. The same ring structure makes it a starting material for pyrrolidones, which move into pharmaceuticals, agriculture, and electronics.
Chemists call it gamma-butyrolactone and jotted it down as GBL in their notebooks for years. Traders sometimes refer to dihydrofuran-2-one or oxolan-2-one. On shipping manifests, you might read bluterolactone or 4-butyrolactone. Each alias travels through the chemical supply chain, but they always describe the same liquid with a stubbornly reactive five-membered ring. Brands in Asia and Europe deliver it under their own labels, sometimes with added certification or regional licensing.
Butyrolactone asks for respect in the lab and factory. Splash it on your skin, and you risk irritation or worse. Occupational guidelines place the safe exposure threshold low, and top shelf labs use sealed, ventilated hoods to prevent accidental inhalation. Storage stays simple: cool, well-ventilated rooms, away from strong acids and bases, and nowhere near open flames. Major agencies—OSHA, REACH, NIOSH—publish strict instructions for handling and emergency cleanup. Every lab tech knows to label secondary containers clearly, and to wear gloves made from nitrile or neoprene. Spills demand fast action with absorbent pads and proper chemical disposal, because drains can’t swallow this compound safely. Chemical plants working at scale install gas scrubbers and leak sensors, not wanting to risk fines or injuries in case of a mishap.
Manufacturers and researchers reach for butyrolactone in every corner of industry. It’s a go-to solvent for paints, inks, electronics cleaning, and surface preparation. In the world of pharmaceuticals, early-stage synthetic work relies on its ring structure to craft more intricate molecules. Polymer manufacturers favor it as a precursor for polyvinylpyrrolidone and other specialty plastics. Cycles in lithium batteries use it as an electrolyte additive, feeding the push for better, safer energy storage. Artists and model builders sometimes mix it into specialty paint thinners, although safety concerns limit its use for hobbyists. Despite its versatility, regulatory restrictions block sales in some regions, where authorities fear its link with illicit GHB synthesis.
Scientists at university and industry labs run new tests on butyrolactone every year, exploring both green chemistry and application in advanced materials. Synthetic chemists tweak process conditions, trying catalysts shot through with rare earth metals or bio-derived reagents, looking for ways to cut emissions and waste. Engineers working on next-gen batteries and supercapacitors study butyrolactone-based electrolytes for safety and performance, sometimes pairing it with ionic liquids or performance additives. The push for biodegradable plastics draws in this compound, as they look for ways to bridge classic chemistry with new environmental standards. In pharmaceuticals, teams chase analogs and derivatives that balance activity and safety—its structural kin, N-methylpyrrolidone, emerges as a less controversial cousin in some research. A drive for transparency and compliance pushes labs to publish both positive and negative data so that the next team learns from each experiment, not just the ones that made headlines.
Medical journals carry decades of animal and human studies on butyrolactone’s toxicity profile. At cellular levels, the compound produces central nervous system depression, causing loss of consciousness or worse at high doses. Chronic exposure leads to liver and kidney strain; acute incidents demand quick medical intervention. Regulatory authorities such as the FDA and EFSA scrutinize new studies for evidence of carcinogenicity or teratogenicity, and set tough occupational exposure limits to keep workers safe. Poison control centers record and catalog misuse incidents, feeding into nationwide databases. Researchers have spent years developing antidotes and first-line treatments—activated charcoal, supportive care, and rapid hospital admission mark the front lines. Industry groups teach workers about exposure routes, symptoms, and emergency response, because accidents rarely grant time for slow decision-making.
Butyrolactone’s future feels balanced between promise and restriction. The green energy transition and electronics miniaturization both demand cost-effective, high-performance solvents and electrolytes, it stands ready among the candidates for continued research. At the same time, lawmakers and regulators eye the black-market risks tied to GHB production, threatening to ratchet down on distribution even further. Research teams look to drop-in alternatives—bio-derived lactones, less toxic ring structures—hoping to keep innovation alive while trimming regulatory headaches. Companies making high-value polymers or next-gen energy storage gear stand to gain from new breakthroughs in synthesis and safety. Public datasets grow as universities and companies publish experimental protocols for greener manufacture, pushing competitors to up their own game. Each year, fresh chemical minds take a crack at butyrolactone, and its story continues to evolve.
Walk through any modern lab, and you’ll spot bottles labeled “butyrolactone.” People outside the chemical industry rarely talk about it, yet this liquid has worked quietly behind the scenes for years. Gamma-butyrolactone, or GBL, runs in many directions—serving medicine, industry, cleaning, electronics, and sometimes, the wrong crowd.
From my time writing about chemicals, I’ve learned butyrolactone rarely gets its due. Most stories focus on buzzier ingredients. Still, GBL can dissolve polymeric resins, strip paint, take apart graffiti, stabilize lithium batteries, and help chemists create new molecules. GBL thrives in solvents, paint removers, and superglue debonders. The stuff even keeps machinery free from stubborn gunk. In short, not many chemicals roll between labs, factories, and tool kits as much as this one.
Look at a pharmaceutical bench, and butyrolactone sits in reaction flasks, quietly making new medicines. Its stable ring structure lets it react with other compounds, opening the door to muscle relaxants and sleep aids. It’s never the last stop, but without it, many remedies fade from reach. Sometimes, GBL’s role even spills over into cosmetics—helping in nail polish removers and lotions not just for its solvent power but for its ability to dissolve stubborn oils safely.
Industrial workers reach for GBL for its brute strength and flexibility. It strips old finishes better than most products, without leaving a harsh smell or damaging delicate parts. It cleans machines. It rinses circuit boards before electronics assembly—a trick I learned from an old repairman who swore by its cleaning punch. Battery makers rely on the compound’s compatibility with lithium, lending stability and safety to phone and car batteries. If you plug in a phone or drive an EV, GBL played its part.
No commentary sticks to science alone. GBL, on the street, turns up in illicit drug circles. It can go through chemical changes to become GHB, attracting attention from law enforcement. Some countries restrict sales or track large purchases. It highlights a tug-of-war that people in chemical safety face—balancing the good GBL can do with real risks. I’ve seen policies tighten for ordinary customers, as lawmakers try to keep industrial users moving forward while shutting out those seeking shortcuts to drug manufacture.
Transparency and control matter here. Chemical suppliers track clients and flag suspicious behavior. Training workers to handle GBL with respect also means fewer accidents. Some manufacturers now blend trace dyes or bitterants, deterring misuse without killing GBL’s usefulness. Open guidelines, government-mandated registers, and updated safety data sheets show up more often in workplaces. These efforts keep the chemical in hands that use it right—even as the world digitalizes paperwork and audits.
Butyrolactone holds a lesson for anyone curious about chemicals. One ingredient can power industries, drive invention, and force tough choices about safety. It shows how one formula, easy to miss, can shape much more than a chemistry set.
Gamma-butyrolactone, better known as GBL, works as a solvent in thousands of factories, found in wheel cleaners and paint removers. This clear, oily liquid once passed unnoticed, barely prompting questions. Then it cropped up as an ingredient in party drugs, and people started to pay real attention.
GBL breaks down inside us to become gamma-hydroxybutyrate (GHB). That’s the same substance that’s caused deaths at raves and private gatherings. I remember reading about a cluster of overdoses just a few years ago—folks took it thinking they’d get a slight buzz and ended up unconscious or worse. GBL goes straight through the stomach lining and hits the brain hard. Even a small dose might send someone to sleep unexpectedly, potently slowing heart and breathing rates. In labs, animals given it saw lung and kidney damage, plus irritation and burns.
Exposure doesn't require drinking it. Breathing in the vapor or getting it on your skin while cleaning wheels or stripping paint can also bring serious health consequences. Even splashing some on your arms at work can irritate or burn the skin. My own time working in automotive shops taught me that nobody recognizes those risks until a burning sensation creeps up or someone’s eyes start watering uncontrollably.
GBL’s easy access worries me. Selling it as a cleaning agent means teenagers and curious adults stumble onto it without understanding what they have. In the UK and across Europe, authorities moved to put real brakes on how it’s sold. Police in the U.S. busted people who bought barrels online to whip up illegal drugs. Public health groups and medical journals have documented hundreds of incidents related to both accidental and intentional exposure, outlining a long track record of overdoses, hospitalizations, and tragic deaths.
In the workplace, chemical safety boards stress using gloves, goggles, and respirators anytime GBL is in play. Factories using it have faced inspections and fines based on reports of workers sent to hospitals from simple contact. Strict reporting and tighter controls helped drop incident numbers in areas where managers took those rules seriously.
Automotive shops, schools, and hobbyists don’t have to rely on GBL. Manufacturers introduced less-toxic alternatives for paint removal and degreasing. Customers can pick up these new products at hardware stores, often highlighted in the safety sheets. I always keep an eye out for newer recommendations—sticking to old habits can land you in a hospital bed if you’re not careful.
Training is key. Employers ought to provide clear safety instructions and detailed product information. I once worked with a guy who simply tossed empty solvent containers without a second thought. A little knowledge about GBL changed his routine—he started using proper disposal bins and swapped for safer chemicals where possible. Even at home, it helps to read the label and respect the warnings instead of assuming an internet product works the same as soap and water.
Using GBL brings risks that reach far beyond the shop floor. Its reputation in the party scene exposed the hidden dangers once thought limited to factories. Safer cleaning products now offer alternatives, and spreading this information cuts down on tragic mistakes. Choosing safety gear, sticking to new formulas, and reading up on the risks all matter—sometimes, a little caution really does make a difference.
Butyrolactone, more formally called gamma-Butyrolactone or GBL, pops up in news headlines for a reason. Quite a few people know it in the context of industrial solvents or as an ingredient in certain cleaning products. The other half of the story—a little darker—touches on recreational misuse and connections with substances like GHB. I learned about GBL in college chemistry, handed a bottle with warning labels plastered everywhere. The teachers were clear about its uses and the dangers, especially if someone swallows it. This stuff pulls double duty, but the risks cut deep, particularly when folks try to use it for effects it wasn’t designed to give.
Even a small amount can flip a person’s world upside down. Nausea hits soon, followed by dizziness and confusion. Some report muscle weakness and wobbly legs, the type of feeling you never forget. Heartbeat skips or drops. Even your breathing can slow, leaving you gasping for air. Using GBL without medical oversight leads to real harm—even death. Some cases involved people falling asleep and not waking up, their bodies shutting down instead of resting. The CDC and other health authorities caution about how quickly the impact can snowball.
Anyone thinking about taking GBL for a high should hear this. Euphoria might come at first, but people commonly feel confusion or heavy anxiety not long after. Panic sets in; with enough exposure, a person may lose touch with reality. As someone who’s watched a friend spiral after misusing chemicals, I can tell you the mess isn’t just in the body—it rips through your thoughts and moods too. Addiction can crop up shockingly fast, echoing stories addicts tell about GHB. Stopping suddenly may lead to shakes, sweating, trouble sleeping, and even seizures. It’s a tough road back.
Some stories come from people with healthy livers who suddenly see issues after GBL use. They mention severe stomach pain and the kind of vomiting that keeps you chained to a bathroom for hours. More digging shows that GBL breaks down into GHB inside the body, stressing the liver and kidneys. For folks with underlying health issues, the risk multiplies. Doctors notice that liver enzymes can skyrocket, a clue that the internal organs are working overtime or losing the fight.
I wish more high school and college programs tackled chemical safety beyond textbook exercises. Peer-reviewed studies back up the need for honest conversations about GBL and other solvents—removing the mystery makes space for real questions and second thoughts. Countries like the US and UK already regulate how people buy and sell GBL, but online sources still dodge rules. Better tracking and stronger enforcement slow down illicit access. For those caught up in misuse, medical experts recommend a combination of counseling and supervised detox to lower the odds of withdrawal complications.
The facts on GBL come backed by toxicology research—this isn’t guesswork. For safety’s sake, folks ought to treat butyrolactone in any form with a level of respect usually reserved for things that can really bite back.
Butyrolactone often shows up in labs and factories, so it helps to treat this chemical with a little respect. Anyone who has worked around chemicals for a few years knows that ignoring storage rules can turn a normal day into an emergency. A clear conversation about why this liquid matters, how it behaves, and what it can do when left unchecked always sticks with me. The best lessons have come straight from the shop floor, watching what happens when rules get bent for the sake of saving a few steps.
Most folks recognize Butyrolactone as a solvent. Fewer talk about its real risks. It slips into the air with little scent, can mess up your lungs, and even eats away at skin if splashed. When kept warm or exposed to moisture, it may break down and form corrosive acids. Years ago, I saw a drum left in the sun on a humid day start to warp and leak along its seams. That taught us to respect not just the chemical, but also the little things like weather, ventilation, and proper labeling.
Storing Butyrolactone right doesn’t call for fancy gear. The best practice I’ve learned is simple: keep it cool, keep it dry, and keep it closed. That means a room with steady air flow, far from heat vents, radiators, or direct sunlight. Any place with less humidity works better because moisture speeds up its breakdown. Flammable safety cabinets do the job for extra protection if you’re sharing space with other volatile chemicals.
Good solid drums or bottles made of high-density plastic or certain metals keep Butyrolactone locked away from the air. Tighten caps right after pouring, and don’t let containers get dusty or corroded. Long ago, we forgot a bottle at the bottom of a shelf, and corrosion on the cap caused a small leak. It’s a bad feeling, smelling an odd sweet odor and not knowing where it started, so now we check seals and replace old containers after a few months.
Official guidelines come down hard on Butyrolactone storage for a reason. Counting on the safety data sheet works—those warnings exist because too many people got sick or hurt before better systems came in. There’s no quicker road to injury than working from memory instead of clear procedures. It’s smart to train anyone handling chemicals like this, and run practice drills for spills or leaks.
Yearly audits of storerooms save both money and time. More than once, we’ve pulled mystery cans off shelves with faded labels, dodging a disaster by just being thorough. Emergency showers and eye-wash stations near storage spots shut down panic before it happens. Management sometimes misses the day-to-day risks, but those working close to chemicals see the faults long before any paperwork does.
No one really wants a story about a spill, a hospital trip, or lost production time. Speaking from experience, small changes in daily habits cut down on those stories fast. Keep personal safety gear close, use only labeled containers, and never guess about a chemical’s age or quality. Reporting problems, even minor ones, nudges the whole crew toward better results.
Storing Butyrolactone right isn’t just following rules—it’s putting people first. A safe storeroom means a safer team, a cleaner record, and no surprises when inspectors show up unannounced. My own work has always gone smoother when I didn’t just check a box, but really made storage part of the routine. That’s how risks shrink from problems to memories.
Most people stumble across butyrolactone because of its role as a solvent and cleaning agent. Industries like electronics and pharmaceuticals turn to it for degreasing, paint stripping, or lab reactions. In my own tinkering days, I saw it at work scrubbing grime off machine parts. It’s got bite, cuts through residue, and gets things gleaming. Its properties aren’t the problem; it’s what people can turn it into that draws attention.
The law struggles to keep up with chemicals that have both everyday uses and serious potential for abuse. The U.S. doesn’t put butyrolactone on its main controlled substances list. So, you’ll find it in some industrial supply catalogs. But that’s not the full story. The Drug Enforcement Administration tracks its use and considers it a “List I chemical.” This means officials keep tabs on big transactions since people use it to make gamma-hydroxybutyric acid (GHB), which the government lists as a Schedule I drug. A dealer selling it for industrial cleaning can operate legally; selling to someone using it for recreational purposes—or knowingly helping that process--will draw federal charges.
States sometimes take a tougher stance. A handful treat butyrolactone like a controlled substance. Big cities or states with histories of GHB abuse might hammer down possession or sale laws even harder. Ordinary people can get caught in the cracks. A legitimate shop owner who stocks it for cleaning supplies could land in legal trouble if state rules shift or documentation isn’t up to date.
Stepping outside the U.S., rules change fast. The European Union monitors butyrolactone under similar “precursor” laws. In Australia, it’s a controlled substance, and just having it can trigger legal trouble. Customs officers flag it at borders, and using it without strict paperwork raises suspicion. People ordering from overseas suppliers sometimes get a surprise visit from law enforcement—this isn’t theory, it’s happened to hobbyists who tried importing for research, and ended up with empty wallets and legal bills.
The consequences go beyond fines. In the last few years, I’ve read about folks facing full-blown federal investigations—not because they intended harm, but because their paperwork was missing or a stranger showed up asking the wrong questions. Officials don’t care if you bought it for home chemistry as a curiosity. Large orders, weird payment methods, or unclear intentions look suspicious.
Clear rules and better awareness could help prevent accidental violations. Regulators ought to communicate with companies and hobbyists, not just lawyers and big industry. Simple guidance and checklists would give buyers peace of mind. Requiring paperwork for large orders, but keeping small quantities available, might balance industry needs and safety concerns.
Butyrolactone sits in a complicated spot: useful for industry, risky for misuse. Anyone thinking of buying or selling it needs to know local and federal law, keep detailed records, and remember this isn’t a chemical for the casual experimenter. Education, smart regulation, and open lines between industry and enforcement agencies will keep communities safe and businesses protected.
| Names | |
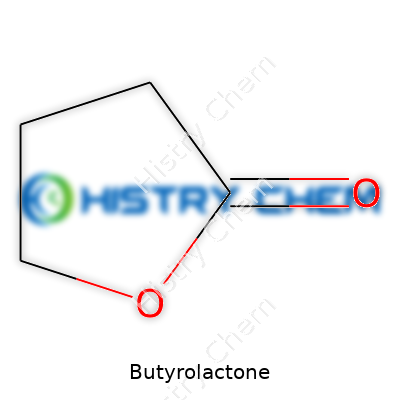
| Preferred IUPAC name | Oxolan-2-one |
| Pronunciation | /ˌbjuː.tɪ.rəˈlæk.təʊn/ |
| Identifiers | |
| CAS Number | 96-48-0 |
| Beilstein Reference | 0007972 |
| ChEBI | CHEBI:31326 |
| ChEMBL | CHEMBL1074 |
| ChemSpider | 4939 |
| DrugBank | DB02160 |
| ECHA InfoCard | 03b1eaf7-8b10-47e6-92cf-0b4bc7093ab4 |
| EC Number | 203-786-5 |
| Gmelin Reference | 8281 |
| KEGG | C06535 |
| MeSH | D001433 |
| PubChem CID | 8050 |
| RTECS number | UF3850000 |
| UNII | 948JXA4Z1L |
| UN number | UN2810 |
| Properties | |
| Chemical formula | C4H6O2 |
| Molar mass | 86.09 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Odor | Faint, weakly aromatic |
| Density | 1.129 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.46 |
| Vapor pressure | 0.97 mmHg (20 °C) |
| Acidity (pKa) | 4.32 |
| Basicity (pKb) | pKb = -1.4 |
| Magnetic susceptibility (χ) | -6.36×10⁻⁷ |
| Refractive index (nD) | 1.446 |
| Viscosity | 1.7 mPa·s (20 °C) |
| Dipole moment | 4.21 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 222.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -589.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2212.8 kJ/mol |
| Pharmacology | |
| ATC code | N07XX32 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation, may cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Danger |
| Hazard statements | H302, H315, H319, H336 |
| Precautionary statements | P210, P261, P280, P305+P351+P338, P337+P313, P403+P233 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 98°C (208°F) |
| Autoignition temperature | 446 °C (835 °F; 719 K) |
| Explosive limits | Explosive limits: 1.5–9.6% |
| Lethal dose or concentration | LD50 oral rat 1540 mg/kg |
| LD50 (median dose) | 1540 mg/kg (rat, oral) |
| NIOSH | DH8225000 |
| PEL (Permissible) | PEL: 100 mg/m³ |
| REL (Recommended) | 500 mg/L |
| IDLH (Immediate danger) | 500 ppm |
| Related compounds | |
| Related compounds |
Gamma-hydroxybutyric acid (GHB)
Gamma-butyrolactone (GBL) 2-Pyrrolidone Delta-valerolactone Butyric acid |