
Chemistry traditions go back centuries, making every new compound a product of ongoing curiosity. 1,4-Benzenedimethanol appeared as chemists explored aromatic compounds and found that transforming terephthalic acid with reducing agents leads to interesting results. Back in the twentieth century, researchers tried different alcohol forms on aromatic rings, which guided early organic synthesis toward compounds like 1,4-Benzenedimethanol. By the time polymer science gained momentum, this compound found its place as a versatile building block, especially as industry demanded new materials with both rigidity and flexibility. This context matters when unpacking how our chemical playbook has changed—driven by both need and ingenuity—with 1,4-Benzenedimethanol as a strong example.
1,4-Benzenedimethanol, better known in labs as p-xylylene glycol or just BDM, stands out as a small molecule with two alcohol groups attached directly to the benzene ring. These simple features lend themselves to a surprising range of uses. Often, this compound shows up in the plastics industry, especially for making polyesters and polycarbonates, but that doesn't cover its full reach. Since it’s both rigid and polar, it gets into resin manufacture, chemical intermediates, surface coatings, and even electronics. One reason users gravitate toward it involves both its predictable structure and easy blending into more complex molecules; this sets it apart from aliphatic diols, offering unique product properties you don't get elsewhere.
Solid at room temperature, 1,4-Benzenedimethanol appears as colorless crystals. It melts reliably above 140°C, which simplifies purification by recrystallization. Solubility in water remains low, but it dissolves well in most alcohols and some ethers. Chemists appreciate its stability under standard storage, since neither the benzene ring nor the methanol groups react quickly unless provoked. This lets it sit on shelves without breaking down, yet those same alcohol groups can jump into esterification, ether formation, or oxidation with the right conditions. Molecular weight sits at around 138 grams per mole, and every storage handler knows to keep it tightly closed—moisture or excess heat can compromise its integrity.
Producers label 1,4-Benzenedimethanol using its CAS number (100-86-7), full name, and purity levels. Industry standards say pure product falls above 98% with only trace water and organic impurities. Packaged as a crystalline powder, delivery comes in sealed, inert-lined drums or containers that block both moisture and oxygen. Regulatory demands require labeling details like batch number, manufacturing date, and detailed safety handling guides. Small changes in technical specification, such as residual solvent or water content, seriously affect downstream processing, especially where consistent polymer properties matter. Once buyers receive a batch, routine GC or HPLC testing confirms these metrics—nobody wants their end product failing quality control.
Industrial preparation of 1,4-Benzenedimethanol starts most often from terephthalic acid or its dialdehyde cousin, terephthalaldehyde. The process turns on hydrogenation, using a catalytic bed—commonly palladium on carbon—under carefully controlled heat and pressure. Experienced chemists mix the reactants in pressurized reactors, add hydrogen gas, and maintain optimal pH to sidestep unwanted side products. In smaller labs, another go-to route takes dimethyl terephthalate, hydrolyzes it to the aldehyde, and then reduces it to the targeted alcohol. These methods yield high-purity material, but they require close monitoring for catalyst poisoning or incomplete reduction. Proper process design turns a challenging synthesis into a routine procedure.
With two benzyl alcohol groups bookending the aromatic ring, 1,4-Benzenedimethanol jumps into plenty of chemical reactions. Esterification stands out: coupling with carboxylic acids or acid chlorides delivers polyester chains, crucial for high-performance plastics. Oxidation steps convert its alcohol groups to aldehyde or acid forms for other intermediate uses. In organic synthesis, the compound turns into various ethers by reacting with alkylating agents—useful when creating surface-active or functionalized materials. Electrophilic substitution on the aromatic ring can bring in more substituents, but sterics and electronics shift these paths depending on conditions. This flexibility opens up new monomer and oligomer families for advanced materials.
In catalogs and research papers, 1,4-Benzenedimethanol goes by names like p-Xylylene glycol, Benzene-1,4-dimethanol, and p-bis(hydroxymethyl)benzene. The basic structure stays unchanged, but translations and supplier branding sometimes create local variations. Large suppliers might mark it under a proprietary label, yet all reference the same CAS number. This helps prevent confusion in international shipping and registration, though quality grades (lab grade, industrial grade) might change packaging or pricing. Anyone scanning technical sheets or MSDS documents will recognize these synonyms—critical for ensuring the right material shows up at your dock.
Safety teams keep a close eye on 1,4-Benzenedimethanol’s handling protocols. The small molecular size makes it less volatile than some aromatics, but dust clouds or residues can irritate eyes and lungs. Standard personal protective equipment includes gloves, goggles, and filtered masks during weighing or transfer. Training covers emergency procedures for spills or accidental ingestion, though industrial users rarely encounter acute health hazards from single exposures. Waste from production follows local and international chemical disposal rules, since downstream oxidation products can raise environmental concerns. Equipment cleaning calls for thorough rinsing, sometimes with ethanol or acetone, to cut down cross-contamination risks. Consistent reviews of safety standards help limit workplace incidents—something no production line manager takes lightly.
Industries harness the versatility of 1,4-Benzenedimethanol in polymer manufacturing and as a chain extender for resins. In polyester and polyurethane production, it controls molecular weight and drives up toughness without making materials brittle. Electronics manufacturers value it for creating high-purity resins that can withstand heat and ultraviolet exposure. In pharmaceuticals, synthetic schemes sometimes employ it as a linker or for protecting groups that need staged removal. Specialty coatings, adhesives, and even compact fluorescent materials draw on its dual alcohol groups to anchor additives and optimize flow properties. The balance between rigidity and processability lets this small molecule punch above its weight, earning it a footprint across several modern technologies.
Research teams never stop pushing boundaries on how to use 1,4-Benzenedimethanol more efficiently or in new settings. University labs study greener, less energy-intensive preparation routes—switching from precious metal catalysts to recyclable or biological systems. Polymer scientists tinker with copolymerizing the molecule for barrier films in food packaging or for high-strength composites in automotive panels. Nanotechnology researchers investigate its potential underlying framework for organic electronic materials, betting on improved conductivity coupled with stability. Conferences regularly feature updates on new blends or derivatives that address emerging challenges, such as recyclable materials or performance in harsh settings. This constant exploration showcases the compound’s versatility and the creative drive within modern chemical sciences.
Toxicology assessments of 1,4-Benzenedimethanol focus on inhalation, skin, and ingestion exposure scenarios commonly encountered in industrial settings. Short-term tests reveal mild irritation at higher concentrations but little chronic harm at occupational levels if workers adhere to established limits. Animal studies report moderate oral toxicity, underscoring the need for controlled handling where accidental contact poses risk. Regulatory bodies encourage rigorous labeling and personal protective measures, especially where dust or vapors might arise from bulk transfer. Environmental pathways—such as improper disposal—prompt aquatic toxicity assessments, pushing for responsible waste management. For now, the compound's safety profile compares favorably to more reactive aromatics, though new data from long-term exposure remains valuable for refining workplace guidelines.
Looking forward, 1,4-Benzenedimethanol stands on the cusp of several industry shifts. Demand for high-performance, recyclable polymers puts it under the lens for innovations in green chemistry. As sustainable manufacturing gains momentum, preparation methods may shift toward bio-based aromatic precursors or more selective catalytic systems. Electronics and clean energy infrastructure might turn to this compound as new adhesive or encapsulating chemistries emerge to improve device lifetime. The trend toward miniaturized, high-durability materials suggests expanded research into its copolymerization capabilities. Each of these directions reflects changing markets and the increasing priority placed on sustainability, making the future of 1,4-Benzenedimethanol a strong bet for ongoing and new applications in advanced materials science.
1,4-Benzenedimethanol might sound like just another obscure compound from a chemistry textbook, but manufacturers and researchers count on it for more reasons than you’d guess. At its core, this chemical makes things stick together better and last longer. Years ago, my own time in a small research lab opened my eyes to the number of ordinary products quietly relying on a handful of sturdy, multipurpose chemicals like this one.
One key way 1,4-Benzenedimethanol stands out is in the world of plastic production. Polyesters, for example, need something that connects molecular chains and gives the end product its needed strength and resilience. This is where 1,4-Benzenedimethanol steps up. It provides those extra links that stop your water bottles from cracking and prevent insulation in wires from crumbling under stress.
I’ve seen factory workers handle granules made with this compound, shaping them into lightweight components used in everything from keyboards to car dashboards. Without these chemical building blocks, designers would have to settle for products that don’t handle hot summers or cold winters as well as they do now.
The reach of 1,4-Benzenedimethanol actually goes past plastics and into fields like medicine. Pharmaceutical firms sometimes use it as part of drug delivery vehicles. These vehicles keep active ingredients stable and release them where the body can get the most benefit. As someone who lost a close family member to a condition that needed steady, reliable drug delivery, I appreciate how advances in chemistry directly transform health outcomes.
Surface coatings have evolved quite a bit, turning simple furniture or flooring into water-resistant, stain-proof, or glossy surfaces. Additives like 1,4-Benzenedimethanol make those coatings grip better and stay intact longer. Families with young kids probably notice this every time juice fails to soak into a kitchen table, or marker lines disappear with a wipe. Durable coatings might look minor in daily life, but they save money and frustration in the long run.
It isn’t all upside, though. Chemicals behind strong plastics and coatings often fail to break down in nature. Waste can pile up, and waterways end up suffering. Studies published by the American Chemical Society highlight concerns about persistence in the environment and the risks that come with it. The task, then, falls to innovators who design recycling processes and eco-friendly alternatives.
One answer rests in “green chemistry”—the push to create compounds that give the same benefits with fewer side effects for the planet. Several university labs now chase biodegradable substitutes. Strong partnerships between academic groups, industry, and government agencies set the bar higher for chemical safety, making real progress possible. Strict regulations already point manufacturers toward cleaner processes, and my own experience with local recycling programs shows public demand plays a part as well.
From holding together plastic bottles to protecting medicine and giving surfaces staying power, 1,4-Benzenedimethanol proves itself in labs, factories, and homes alike. Chemical innovation rarely stands still. Each push for safer materials, better recycling, and smarter chemistry just writes another chapter in the story of compounds like this one.
1,4-Benzenedimethanol stands out in the world of chemistry, not just as a name that stretches across a blackboard, but as a compound with potential that flows into many corners of science and industry. It brings together a benzene ring, solid and stable, with two swinging —CH2OH groups, each anchored on opposite ends. The chemical formula is C8H10O2. Chemists and engineers keep this structure in mind, especially in polymer science and specialty manufacturing.
Looking at C8H10O2 gives a picture of eight carbons, ten hydrogens, and two oxygens. In practice, each molecule holds a benzene ring as the backbone (six carbon atoms arranged in a familiar hexagonal shape) and a methanol (—CH2OH) at the para positions, which means each sits across from the other. That orderly structure gives 1,4-Benzenedimethanol its stability, predictability, and appeal in creating stronger, longer-lasting materials.
Polymers touch nearly everything in daily life, from water bottles to athletic gear. 1,4-Benzenedimethanol acts as a building block in the creation of polyester resins and other engineered plastics. The alcohol groups (—CH2OH) on either end make it reactive and useful in condensation reactions. This flexibility means companies prefer it for customizing polymers, aiming for better strength and improved weather resistance. The push for more sustainable and durable materials turns attention to compounds like this.
Beyond plastics, 1,4-Benzenedimethanol finds a place in pharmaceuticals, dyes, and even fuel additives. By connecting its two “arms” to different chemical partners, chemists spin off new products that have practical uses in real life. Scientific literature documents its flexibility and efficiency — sometimes boosting yields, streamlining processes, or reducing waste compared to older alternatives.
A lot rides on managing chemicals safely. 1,4-Benzenedimethanol does not give off strong fumes, nor does it carry the worst labels in hazard ranking charts, but that does not mean lab workers let down their guard. Anyone handling it in a manufacturing setting should respect protective guidelines. Reliable sources like the National Center for Biotechnology Information confirm low acute toxicity, but repeated exposure or improper disposal could still threaten ecosystems. Disposal calls for established protocols and attention to wastewater streams.
The path from raw chemical to finished product grows more traceable each year. Consumers want to know what compounds shape the things they buy, and manufacturers respond by disclosing chemical use through regulations like REACH in Europe and TSCA in the US. Accurate chemical formulas like C8H10O2 keep the supply chain open, letting experts track, monitor, and improve what flows into the environment.
Staying transparent about chemical composition, safety data, and sourcing protects both people and the planet. Researchers and process engineers search for cleaner, safer, and more sustainable ways to use compounds like 1,4-Benzenedimethanol, often sharing data openly in respected journals and public databases. Progress relies on clear facts, solid peer-reviewed evidence, and respect for science at every stage of a compound’s journey from lab bench to real-world application.
1,4-Benzenedimethanol comes up in conversations about industrial chemicals. It’s a solid that functions as a building block for things like plastics, coatings, and even pharmaceuticals. You’ll spot it on a chemical label as C8H10O2. Factories use it to get the right texture in resins or to bind things together in polymer mixes. For most folks, direct exposure isn’t common—unless you’re working with industrial raw materials or chemical manufacturing.
Questions pop up about the safety of any chemical in large-scale use. People want to know: is handling 1,4-Benzenedimethanol risky? Data from regulatory agencies, including the European Chemicals Agency and US National Library of Medicine, shows contact with pure forms can cause irritation. Dust from the powder form might hit your eyes or lungs with a sting, especially if you’re not using gloves or a mask.
From my time working near chemical storage areas, no one liked handling powdered chemicals without proper gear. Even minor skin contact caused dry patches. The main risk pops up with repeated or long-term exposure during manufacturing, not from finished products made with it. If the air isn’t filtered or spills aren’t handled well, breathing in particles brings coughing fits or headaches.
So far, cancer links haven’t come up in scientific literature for 1,4-Benzenedimethanol. That fact separates it from high-profile chemical threats like benzene itself, which carries a serious risk for leukemia. Studies haven’t linked the “dimethanol” form to reproductive damage or organ toxicity in humans. The EU and US labeling rules use the “irritant” warning, not the skull-and-crossbones.
No one should mistake that for a free pass. Safety comes from habit—wearing gloves, goggles, and keeping air flowing, especially where this chemical gets heated or ground. That’s the difference between high school chemistry class and a factory floor. The Chemical Safety Board records several cases of mild poisoning in people cleaning up leaks or breaking up dried residue.
In every workshop I visited, one constant pops up: clear safety rules and enforcement checklists make a real difference. Workers benefit from regular training, and plant managers never skip labeling or lockout procedures. For 1,4-Benzenedimethanol, air filtration and vacuum cleaning help avoid clouds of dust. Anyone handling bulk stock takes routine health checks, mostly checking for eye or lung problems over time.
Regulators sometimes lag behind the latest research, so worker-driven committees push for extra caution. Hearing stories at industry meetings, one lesson stood out: accidents go down when everyone feels empowered to halt sloppy storage or demand a new pair of gloves. That builds confidence — not just for staff, but for people living near these operations.
Science hasn’t found major toxicity in small doses, but letting up on protective steps usually ends in regret. Today, fewer plant workers wind up in the nurse’s office, thanks to transparent record keeping and stricter audits. By learning from past mistakes—like skipped ventilation or ignored warnings—companies make sure 1,4-Benzenedimethanol fills its industrial role without putting people at unnecessary risk.
The name 1,4-Benzenedimethanol may not spark the average person’s interest, but for those handling chemicals daily, its straightforward storage needs demand respect. This solid—white crystals, slightly sweet smell—shows up in labs and factories for all sorts of uses, especially where polymer production gets serious. A lot can go wrong with chemicals if corners get cut. I’ve seen too many stories in the news about accidents that started with sloppy storage choices, and those stories rarely end well.
Moisture and heat pull trouble when paired with 1,4-Benzenedimethanol. It stays stable at room temperature, but humidity breaks down the integrity of the crystals over time. Heat increases risks, making the compound more likely to decompose or release vapors that shouldn’t drift through your workspace. Storing this chemical in a dry, cool place is just common sense.
Safety data from the world’s best labs back this up. The compound hasn’t earned a spot on explosive or highly toxic lists, but mishandling still means ruined product at best, or toxic fumes at worst. Stainless steel shelves—lined, not bare—inside a well-ventilated, temperature-controlled room help dodge these pitfalls. Avoid cardboard boxes or open containers. I once worked in a facility where water from a leaking air conditioner dripped unnoticed into a storage closet. Within a week, the costs from spoiled material and safety checks far outweighed the expense of proper humidity control.
Throwing every chemical onto the same rack is a rookie mistake. 1,4-Benzenedimethanol has low flammability, but mixing in strong oxidizers changes the game. Cross-contamination happens fast and can trigger reactions that nobody in their right mind wants to babysit. Clear labels matter—a faded marker on a jar can lead to confusion and dangerous mixes. Invest an extra five minutes updating labels instead of hours cleaning up a spill. OSHA’s guidelines push for this level of clarity because mistakes grow expensive—sometimes deadly—without it.
I’ve had to open too many doors to stuffy chemical storage rooms, catching a nose full of questionable air. I remember a time, fresh out of college, when a basic exhaust system saved a whole shift from headaches after a minor leak. Fume hoods and exhaust fans don’t belong just in the movies; they lower health risks and keep air quality up. 1,4-Benzenedimethanol doesn’t go airborne easily, but in a confined, unventilated space, even small emissions stick around.
Bulk chemicals deserve the decent treatment that storage experts preach about. Durable, airtight containers, proper labeling, regular inspections, and well-trained staff don’t just protect product—they protect people. Training needs to emphasize real consequences, not just rules on paper, making it a habit to check seals, scan thermometers, or swap out damp-damaged cartons.
If a chemical stocks room relies on luck, trouble’s only a matter of time. Consistent routines and basic investments keep 1,4-Benzenedimethanol safe, pure, and ready for its next use. Simple steps win out over cost-cutting every single time.
1,4-Benzenedimethanol stands out in the landscape of organic building blocks. Companies rely on this compound to create polymers, resins, and specialty chemicals—products that end up in everything from electronics to automotive parts. So, having access to reliable synthesis methods makes a difference not just for chemical manufacturers but for researchers and industries that need consistency and predictability in their raw materials.
The most direct route starts with terephthalaldehyde. By running a reduction—usually with sodium borohydride—chemists convert the aldehyde groups to the sought-after benzyl alcohol units. This method stands out for its selectivity; side products rarely complicate the process when carried out with controlled conditions.
There’s an alternative approach, too. Some practitioners rely on the hydrogenation of dimethyl terephthalate or terephthalic acid, using specialized catalysts like Raney nickel or palladium on carbon. The high-pressure hydrogenation setup can be expensive for smaller labs, but the scalability attracts larger operations where volume matters more than convenience.
Another method, less common but clever, involves the reduction of 1,4-dibromomethylbenzene. Using lithium aluminum hydride, this path usually turns out quite efficient, though handling LAH demands respect for its reactivity and the dry conditions it requires.
Small labs and startups have to weigh cost, skill, and safety. Not every group has experience with handling hydrides or setting up hydrogenation. The route from terephthalaldehyde and sodium borohydride gives people an affordable and approachable option, even for those running a fume hood in a teaching lab. A scale-up to manufacturing changes the math, and then hydrogenation starts looking smarter for throughput.
From personal lab bench mistakes, I learned the hard way that sodium borohydride wastes no time generating heat and hydrogen. Overzealous addition routines lead to foaming and sometimes a scary moment or two. Careful slow addition, cooling, and choosing the right solvent—these steps keep things under control.
Hydrogenation presents other risks. High-pressure hydrogen and metal catalysts have no forgiveness for shortcuts. A leak or catalyst dust can set up an accident. That’s why most folks only attempt this with the support of trained process chemists, safety equipment, and protocols that leave nothing to guesswork.
Sustainability now shapes the conversation around chemical synthesis. People seek less toxic reagents and catalysts that last through multiple uses. Some researchers have looked at enzymatic reductions and milder reagents, hoping to ditch dangerous alkali metals or transition metals for something friendlier to both worker and planet.
Academic groups in Germany and Japan reported success with immobilized enzyme catalysts and greener reducing agents, cutting down on waste and harsh solvents. Adoption in industry stays limited for now, but these innovations suggest a future where synthesis gets cleaner and safer without sacrificing yield.
The field keeps evolving, learning from mishaps and advances alike. Keeping workers safe and waste streams manageable depends on learning from both established industry procedures and emerging green chemistry research. Training, respect for hazardous reagents, and willingness to try new technologies build a foundation that serves both chemistry and the people behind it.
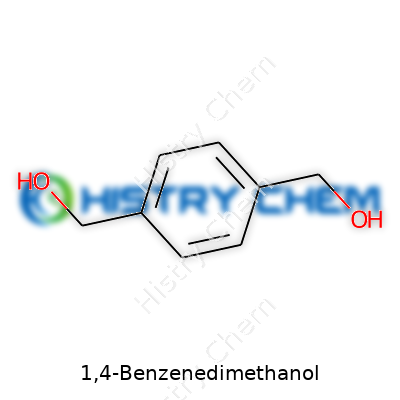
| Names | |
| Preferred IUPAC name | benzene-1,4-dimethanol |
| Other names |
1,4-Benzenedimethanol
p-Xylylene glycol p-Bis(hydroxymethyl)benzene 1,4-Bis(hydroxymethyl)benzene p-Xylenedimethanol Terephthalyl alcohol 1,4-Dimethylolbenzene |
| Pronunciation | /ˌwʌn.fɔːr.bɛnˈziːn.daɪˈmɛθ.ə.nɒl/ |
| Identifiers | |
| CAS Number | [100-14-1] |
| Beilstein Reference | 626089 |
| ChEBI | CHEBI:140411 |
| ChEMBL | CHEMBL36276 |
| ChemSpider | 7234 |
| DrugBank | DB04248 |
| ECHA InfoCard | 03b6c8ec-2c49-424e-8fd0-1a8c8dbf171b |
| EC Number | 204-626-7 |
| Gmelin Reference | 60677 |
| KEGG | C02571 |
| MeSH | D003883 |
| PubChem CID | 7218 |
| RTECS number | CU5950000 |
| UNII | J3G4918547 |
| UN number | UN 2811 |
| CompTox Dashboard (EPA) | DTXSID4020706 |
| Properties | |
| Chemical formula | C8H10O2 |
| Molar mass | 138.17 g/mol |
| Appearance | White crystalline solid |
| Odor | Odorless |
| Density | 1.18 g/cm3 |
| Solubility in water | slightly soluble |
| log P | 0.28 |
| Vapor pressure | 0.000046 hPa (25 °C) |
| Acidity (pKa) | 14.98 |
| Basicity (pKb) | 15.19 |
| Magnetic susceptibility (χ) | -69.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.554 |
| Viscosity | 13 cP (20°C) |
| Dipole moment | 1.68 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 207.8 J/mol·K |
| Std enthalpy of formation (ΔfH⦵298) | -327.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3256.7 kJ/mol |
| Hazards | |
| Main hazards | Causes serious eye irritation. Causes skin irritation. May cause respiratory irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P280-P305+P351+P338-P337+P313 |
| NFPA 704 (fire diamond) | 1,1,0 |
| Flash point | > 185 °C |
| Autoignition temperature | 410 °C |
| Lethal dose or concentration | LD50 (oral, rat): 3,240 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2,240 mg/kg |
| NIOSH | BQ8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/m³ |