
Many chemicals come and go in the industry, but few carry a history as rich as 1,2,4-butanetriol. Chemists first synthesized it over a century ago, fascinated by its structure and the multiple hydroxyl groups it possessed. Before digital recordkeeping, researchers relied on handwritten notes, glass equipment, and a bit of bravado to isolate and characterize new compounds. They quickly realized that this triol holds promise for both practical and research applications. Over the decades, new technologies in synthesis and purification helped refine production and made larger quantities possible. 1,2,4-butanetriol’s importance increased during research surges in propellant chemistry and specialty polymers, especially as scientists searched for alternatives to more volatile compounds. As scientific understanding deepened, interest in its niche but crucial roles continued to grow.
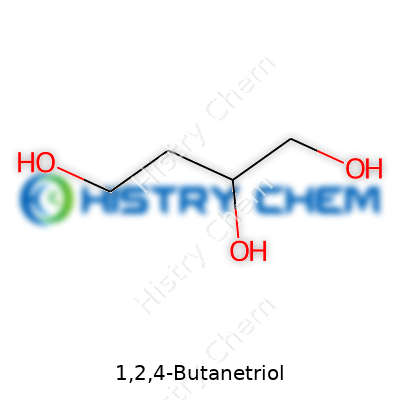
1,2,4-Butanetriol is an organic compound with the formula C4H10O3, sporting three hydroxyl groups arranged uniquely along a four-carbon backbone. Its clear, colorless appearance might seem unremarkable, but its chemical behavior truly distinguishes it. Labs and manufacturers recognize its value in crafting more complex molecules, intermediates for pharmaceuticals, and energetic materials. Controlled handling and measured dosing remain a constant, given its application in sensitive synthesis processes.
Look at 1,2,4-butanetriol, and you may notice its sweet odor and high viscosity, more pronounced than simpler diols. With a boiling point around 285 °C and a melting point near 21–22 °C, it stays liquid across a wide temperature range, making it easy to work with in industrial and laboratory settings. Its density hovers at 1.16 g/cm3. Three reactive hydroxyl groups allow it to build strong hydrogen bonds, giving rise to high solubility in water and alcohol. This unique combination, along with chemical stability under normal lab conditions, offers both opportunities and challenges in storage and transportation.
Regulation continues to shape how 1,2,4-butanetriol is labeled and quality-checked. Purity levels typically reach above 98%, with water content kept under 0.5%. Labs report detailed analysis on impurities because those trace elements, especially aldehydes or heavy metals, can change downstream results. Its CAS number is 3068-00-6. On product labels, manufacturers highlight batch number, purity, handling precautions, and expiration. Certificates of analysis often accompany shipments, supporting transparency and traceability. These details help users match the product’s properties to their intended applications, aiding regulatory compliance and lab reproducibility.
Researchers try different routes for synthesizing 1,2,4-butanetriol, but the two main methods dominate: chemical reduction and fermentation. Traditionally, chemists rely on sodium borohydride reduction of succinaldehyde, a process demanding careful control, but producing reasonable yields. Fermentation offers an alternative, using genetically engineered microorganisms and renewable resources to convert sugars into the triol. This bio-based approach supports the push toward greener chemistry, cutting down on hazardous waste and raw material costs. Industrial synthesis sometimes blends both methods, balancing cost, efficiency, and environmental impact.
With its three reactive hydroxyl groups, 1,2,4-butanetriol stands out for versatility. Chemists can transform it into esters, ethers, nitrates, and even cyclic compounds with relative ease. Nitration produces butanetriol trinitrate (BTTN), a key component in propellant and explosive mixtures, offering lower volatility and higher thermal stability compared to nitroglycerin. Esterification yields surfactants and specialty intermediates. The triol’s structure also allows combinatorial chemistry, opening routes to pharmaceuticals and new polymers. Its chemical landscape rewards creativity from both academic and industry scientists, who value a building block that supports many routes.
Searching for 1,2,4-butanetriol uncovers a list of alternative names including trimethylene glycol, 1,2,4-tetramethylene triol, and butanetriol. Trade names may pop up in supplier catalogs, with varying purity and intended use. Despite the differences in labeling, the underlying chemical structure stays consistent, helping professionals match the right product for specific applications. Knowledge of these synonyms helps navigate regulations and procurement, especially when switching suppliers or moving between international markets.
Any discussion about using 1,2,4-butanetriol requires attention to robust safety practices. Contact may cause mild irritation to skin or eyes, and while its toxicity remains lower than many common solvents, standard PPE remains the rule in responsible labs and factories. Good ventilation and spill management protect workers from inhalation risks. Given its energetic chemistry, strict storage guidelines address fire and contamination hazards. Safety Data Sheets accompany all shipments, offering clear instructions in case of emergencies. Facilities that handle larger quantities invest in training, emergency preparedness drills, and up-to-date equipment—prevention, not reaction, anchors the approach to operational safety.
Few chemicals cross industry boundaries as easily as 1,2,4-butanetriol. Defense industries appreciate its use in synthesizing energetic materials, particularly BTTN, a safer propellant alternative thanks to its higher flash point and improved handling. On the pharmaceutical front, its backbone finds a place in synthesizing cardiovascular drugs and antibiotics. Polymer manufacturers experiment with it as a monomer for biodegradable plastics and surfactants. Research teams, too, use 1,2,4-butanetriol to probe catalytic reactions or develop new ligands. This flexibility and staying power reflect its combination of reactivity, safety, and economic viability, giving it a firm foothold in specialty and bulk chemistry.
Curiosity drives advancement. Labs across academia and industry continue searching for novel applications and better synthesis routes for 1,2,4-butanetriol. The push for green chemistry redirects research attention to fermentation and bio-based pathways, aiming to lower carbon footprints. Drug designers investigate its use in chiral auxiliaries and new drug scaffolds, trying to exploit its structural quirks for better efficacy and bioavailability. Polymer scientists chase high-performance materials with tailored flexibility or biodegradability. Collaborations between chemical engineers and biotechnologists keep expanding the boundaries, showing what can happen when old molecules meet new ideas.
Careful toxicology studies underpin all industrial chemicals, and 1,2,4-butanetriol has not escaped scrutiny. Animal studies show that high doses can cause low-level kidney and liver disruption, but standard use levels in occupational settings keep exposures well under these thresholds. Chronic exposure has not triggered major regulatory concern, likely due to its low volatility and minimal skin absorption. Metabolic studies examine how the body processes and excretes this triol and flag areas where vulnerable populations could experience higher risks. Modern industry leans on this research to guide exposure limits and safety engineering controls, demonstrating that good science protects not just workers, but neighborhoods and ecosystems as well.
As global industries move toward sustainability and efficiency, 1,2,4-butanetriol stands ready for reinvention. Developers look at renewable feedstocks and advanced catalysis to produce it cleaner and cheaper. Energetics experts turn to its trinitrate form as regulations limit riskier explosives. Polymer researchers use it to build next-generation plastics that balance performance with environmental responsibility. The blend of accessible chemistry and solid industrial experience supports further investment and academic innovation. For people who appreciate chemistry’s role in societal progress, compounds like 1,2,4-butanetriol demonstrate how specialized knowledge and responsible management lay the foundation for safer and more effective technologies.
Everyone hears a bit about chemicals in news reports, then forgets the names. Every once in a while, a chemical like 1,2,4-Butanetriol sneaks into headlines—usually not for ordinary reasons. This compound shows up in both peaceful and troubling places. In the lab, it acts as a handy tool for chemists. On the darker side, headlines mention it because it helps cook up energetic materials—meaning explosives, including military-grade stuff like butanetriol trinitrate (BTTN).
Let’s start with the basics in manufacturing. Companies use 1,2,4-Butanetriol as a building block for tough resins, fancy plastics, and specialty coatings. For anyone who paints furniture, deals with high-end electronics, or even uses car parts, this chemical plays a part in making the materials last longer and stay smooth. It acts as a polyol—basically, a molecule that gels together components in polyurethanes and polyester resins. These materials end up in coatings for cars, flexible foams, adhesives that outlast cheap glue, and wires wrapped in insulation made to handle tough environments.
Anyone who’s ever tried patching a wall, only to have it peel months later, learns the hard way about the value of good chemistry. 1,2,4-Butanetriol brings properties that keep coatings from cracking and hold plastics together over time. Manufacturers keep turning to it when they want their products to hang in there, not break down by the first heatwave.
Now, not every chemical becomes news because it helps with paints and plastics. 1,2,4-Butanetriol makes headlines because governments watch its movement around the world. This single chemical forms the backbone for BTTN, a liquid explosive with a high reputation in military circles. Factories producing propellants for missiles, rockets, and artillery shells depend on this stuff, especially since it works in low temperatures and doesn’t freeze as easily as nitroglycerin.
This is why 1,2,4-Butanetriol drew attention beyond industrial circles. Back in 2003, international authorities put it on the list of key precursor chemicals. The concern: criminals or rogue states finding ways to sidestep rules, buying up this material to make explosives instead of paints or plastics. The United States and China put regulations in place because of credible risks. Any business selling, shipping, or buying the substance has to keep records, report suspicious activity, and play by the rules. There’s no way around this if a company wants to keep its license and reputation.
As someone who’s worked in manufacturing, I learned that safety and tracking beat cutting corners every time. Traceability—knowing who handles every barrel, every shipment—matters. Companies started using better tracking systems and digital logs, not only to stay in line with the law but because one slip can wreck years of work.
International cooperation pushes these efforts forward. The Organisation for the Prohibition of Chemical Weapons (OPCW) steps in when something looks off. Routine audits mean that only trusted, vetted players can deal with sensitive chemicals like this.
So, while 1,2,4-Butanetriol keeps showing up in news stories, its place in modern manufacturing stays secure, as long as companies, regulators, and workers keep a sharp eye out and don’t look for shortcuts. That kind of vigilance makes a difference you can’t see until something goes wrong.
1,2,4-Butanetriol pops up in chemistry discussions as a clear, slightly viscous liquid. Some folks use it in the production of energetic materials, like explosives and propellants, and it can show up as a building block for pharmaceuticals. It is neither rare nor a novelty in certain manufacturing plants. Still, questions keep emerging about its safety. Many workers, neighbors, and even the end-users deserve a straight answer: does it pose hazards or toxicity risks?
Laboratory research and occupational safety guidelines reveal a mixed picture. 1,2,4-Butanetriol does not spark headlines for severe toxicity, but that never means it’s harmless. Researchers have seen that swallowing or inhaling high concentrations produces adverse effects, including central nervous system depression and respiratory difficulties. The Occupational Safety and Health Administration (OSHA) and NIOSH don’t list it as a major hazard, but the lack of a strong warning doesn’t mean relaxation is in order. Studies on animals show that at high doses, this stuff can cause nervous system problems, liver changes, and weight loss. Regular workers handling gallons of the stuff shouldn’t treat it like water.
In my lab days, nothing teaches respect for chemicals like a persistent cough after a fume hood malfunctions. Even when something flies under the radar for its toxicity, repeated handling and lack of care can lead to trouble. Some cases share a pattern: unknown or under-reported risks result in headaches, skin reactions, nausea, or just an uneasy feeling that something’s not quite right. Over time, those small effects add up.
Plants that use 1,2,4-Butanetriol have a responsibility to put real safeguards in place. Good ventilation matters. Personal protective equipment—like gloves and goggles—should be non-negotiable. Emergency plans must cover accidental leaks or spills, since the chemical can irritate eyes and skin. Time after time, I’ve seen problems arise from shortchanging safety for convenience. The extra ten minutes putting on gear never looks like a waste after someone avoids a trip to the hospital.
It’s not just those inside the factory who could be affected. If 1,2,4-Butanetriol leaks into groundwater, it may not be as toxic as, say, hexavalent chromium, but we don’t always know how breakdown products will behave. Water contamination leads to bitter local conflicts, and too many communities have learned the hard way that “low risk” doesn’t mean “no risk.” This is especially true when oversight gets lax and spill reports slip through the cracks.
Transparency shifts the balance from confusion to action. Safety data sheets need plain language, not just legal disclaimers or technical jargon. Regular training beats a dusty, forgotten binder. Sharing findings between companies and local hospitals helps track long-term health patterns. Regulators, plant managers, and workers benefit from hearing the same facts, not a different story for each stakeholder.
Only vigilance and honesty keep a chemical like 1,2,4-Butanetriol from causing harm. People deserve information that’s not sugarcoated. Routine monitoring of both air quality and worker health catches trouble before it grows. Chemical safety improves with small, daily choices, not just grand declarations or rare audits. In the end, smart handling and shared knowledge do far more to keep people safe than hoping a hazard never turns real.
Looking up 1,2,4-butanetriol in a chemistry book brings you face to face with the formula C4H10O3. Nothing fancy, just four carbons, ten hydrogens, and three oxygens. At a glance, it reminds some folks of other small alcohols, but the three hydroxyl groups tucked at positions one, two, and four give it special character.
Textbooks toss around polyols like they're a dime a dozen—think glycerol or erythritol. 1,2,4-Butanetriol belongs in the same neighborhood but comes with its own quirks. The three alcohol groups let it find jobs in places where a more basic alcohol wouldn’t do. In the lab, I've watched it dissolve faster than some others because those -OH groups love water. This helps when creating solutions that need hands-on mixing instead of leaving you with stubborn solids at the bottom of your beaker.
Most folks won’t see 1,2,4-butanetriol on a grocery shelf. Step into a chemistry lab or a specialty factory, you might see it on stock lists. It’s become a little famous in the world of propellants for rocket fuel. It plays a role in making butanetriol trinitrate—sometimes called BTTN. This compound is valued for being less sensitive than nitroglycerin. You get power without the same danger level. That difference saves lives for those who handle or transport fuels. The military and space sectors count on lower-risk compounds, and substances like these give them more options.
Beyond propellants, niche chemical syntheses also rely on these flexible alcohols. I’ve seen researchers reach for it when they need to make something with evenly spaced oxygens or need to build other complex molecules from scratch. The presence of three reactive centers, spaced farther apart than in glycerol, gives it a distinct edge when building longer or more branched organic molecules. Even within pharmaceutical labs, its flexibility lets it act as an intermediate for specialty drugs, opening doors for creative synthesis.
Chemicals like this demand respect. Every lab coat I know keeps Material Safety Data Sheets close. While not the most hazardous thing out there, its proper handling and storage prevent problems down the line. Spills, poor ventilation, or careless mixing can lead to headaches—quite literally—and bigger messes in a work zone.
There’s a growing push to clean up how chemical companies make and use substances like 1,2,4-butanetriol. Traditional processes start with petroleum feedstocks, which means higher carbon footprints and environmental headaches. Some creative teams now work on greener methods, like biobased syntheses. Lowering energy use, using fewer harsh chemicals, and improving yields all add up when trying to shrink waste. More labs keep an eye out for updates—for both environmental and budget reasons. Less reliance on oil also means more stability in the face of unpredictable global markets.
Understanding C4H10O3 gives us more than a nice chemical name. It ties into real work done in fuels, pharmaceuticals, and greener chemistry. Taking care of safety and looking for better synthesis routes help both people working with it and those living around production sites. By putting care into how this molecule is made and used, society gets the benefits without extra risks or unwanted footprints on the planet.
1,2,4-Butanetriol seems like just another clear liquid on the shelf, but people who spend time in labs or warehouses know that looks can be deceiving. This chemical shows up in everything from resins to explosives. Its value in the production chain is high, but so are its hazards; nobody coming home from a shift wants to talk about a spill, a fire, or worse.
Unlike table sugar or even ethanol, 1,2,4-Butanetriol brings its own risks to the game. If you leave it too close to a heat source, it goes from harmless to dangerous fast. Its flash point is just above 150°C, which means higher temperatures start to get risky. The fumes aren’t pleasant either; repeated breathing can irritate the lungs and mess with your mood. Gloves and goggles come out for a reason.
People ask, “Couldn’t this just go on a regular storeroom shelf?” That’s missing the point. Companies that ignore proper storage deal with lost product, angry inspectors, and sometimes even serious emergencies. Keeping 1,2,4-Butanetriol in a cool, dry room with plenty of circulation isn’t overkill—that’s good practice built on hard experience. At my old job, one tank got too warm during shipping. The stench was unforgettable, and the cleanup took hours; money was the least of our worries.
Keep containers tightly sealed. Some folks leave lids loose, thinking it lets gas escape, but that only invites contamination and moisture. Water mixing in ruins the batch, and the cost climbs quickly.
Steel drums with corrosion-resistant coatings make a difference. Standard iron or aluminum turns brittle with time, and leaks sneak up on the night shift. Polyethylene containers work well for small volumes. Labeling has to be clear, since accidents often happen when people grab the wrong bottle in a rush.
Chemicals rarely cause disasters all by themselves. The real risk comes from ignoring best practices. Daily checks on storage temperature, double-checking that the ventilation fans are running, and keeping a log of inspections create a safety net. It only takes one overlooked step to cause an issue no one wants to deal with.
Most facilities I’ve seen keep fire extinguishers within arm’s reach near the chemical storage area. Not every foam works on this chemical; dry powder or CO₂ extinguishers should always stand by. Sprinklers add a layer of protection, but nothing replaces quick thinking and a practiced team.
Industry reports and safety boards publish incident breakdowns every year. Lessons from those stories show patterns: slips in labeling, rush jobs, ignored temperature alarms. Decision makers who treat safety as a side issue set people up for failure.
At the end of the day, storing 1,2,4-Butanetriol well pays off. You don’t end up with damaged stock, emergency calls, or costly downtime. Safety isn’t just paperwork; for chemicals like this, it’s the difference between a normal shift and a nightmare that follows you home.
Factories and chemical plants rely on dependable building blocks to turn raw materials into the products seen on store shelves. 1,2,4-Butanetriol, a colorless, water-soluble liquid, finds its way into a surprising number of applications. I’ve handled industrial chemicals before and seen how decisions are made around cost, safety, and performance. Producers stick with 1,2,4-Butanetriol because it pulls its weight on several levels.
This chemical plays a major role in military and aerospace. Factories use it to make butanetriol trinitrate (BTTN), which ends up in rocket propellants and explosives. The U.S. Department of Defense notes BTTN gives improved energy and lower sensitivity than older compounds. That reduces risks for workers and can boost reliability in demanding environments. With global defense spending above $2 trillion, materials that offer safety and efficiency win contracts. Regulations keep tightening, so suppliers need safer, compliant alternatives. 1,2,4-Butanetriol meets these demands and keeps its foothold.
Drug companies often search for molecules they can tweak to create effective medicines. 1,2,4-Butanetriol steps into the pharmaceutical world as a starting material for a variety of antiviral and cardiovascular drugs. Scientists transform it through selective chemical reactions, creating active pharmaceutical ingredients like statins and beta-blockers. Using a reliable intermediate saves time, ensures product consistency, and lets teams focus on scaling up. I’ve seen tight scrutiny from health regulators over purity, so manufacturers count on intermediates with a proven track record.
In the hunt for stronger plastics and superior performance coatings, 1,2,4-Butanetriol works as a crosslinking agent. Adding it to polymer systems adjusts flexibility and strength, which matters for technologies like adhesives, paints, and high-performance plastics. When a company develops a new insulation or automotive part, engineers experiment with different crosslinkers to hit the right physical properties. I’ve watched lab teams run durability tests, checking if a new resin can outlast sunlight, moisture, and rough handling. If a material stands up to these tests, it often works its way into products used every day.
Not every use of 1,2,4-Butanetriol shouts “industry.” The same molecule creates synthetic intermediates for flavors and fragrances, often found in household products and cosmetics. In these cases, companies use the chemical’s backbone to build more complex molecules safely and consistently. Safety matters, since anything added to a food or skin product needs a clean chain of supply with full traceability. The International Fragrance Association sets tough standards on chemical purity, and proven intermediates smooth compliance.
Handling 1,2,4-Butanetriol calls for careful management, like any industrial chemical. Workers need training to avoid exposure, and spills must be contained to protect local ecosystems. Finding greener production routes has become important, so research into bio-based sources continues. Factories adopting closed-loop systems and greener solvents cut down risks and waste. The chemical industry still has room to improve, but experience shows those who invest in safety, transparency, and cleaner processes come out ahead in the long run.
Global demand for specialty chemicals keeps shifting as new technologies, regulations, and supply chain changes shape the market. Companies that innovate around proven molecules like 1,2,4-Butanetriol keep their lead by investing in safety, quality, and sustainability. In my experience, those values resonate far beyond the factory floor—they help build trust with partners and support a more responsible industry.
| Names | |
| Preferred IUPAC name | Butane-1,2,4-triol |
| Other names |
Butane-1,2,4-triol
1,2,4-Butantriol Trimethylolethane Homopentaerythritol |
| Pronunciation | /ˌwʌnˌtuːˌfɔːr.bjuːˈteɪn.triːˌɒl/ |
| Identifiers | |
| CAS Number | [3068-00-6] |
| Beilstein Reference | 1720240 |
| ChEBI | CHEBI:16711 |
| ChEMBL | CHEMBL161455 |
| ChemSpider | 2155 |
| DrugBank | DB02802 |
| ECHA InfoCard | 03c0919b-5e58-4d0e-99c7-c1c60c848a50 |
| EC Number | EC 203-036-9 |
| Gmelin Reference | 1901930 |
| KEGG | C01716 |
| MeSH | D017174 |
| PubChem CID | 8108 |
| RTECS number | EY5950000 |
| UNII | 85G2U24K82 |
| UN number | UN3439 |
| CompTox Dashboard (EPA) | DTXSID7036792 |
| Properties | |
| Chemical formula | C4H10O3 |
| Molar mass | 122.12 g/mol |
| Appearance | Colorless to pale yellow hygroscopic liquid |
| Odor | Odorless |
| Density | 1.13 g/cm³ |
| Solubility in water | miscible |
| log P | -0.78 |
| Vapor pressure | 0.02 mmHg (20°C) |
| Acidity (pKa) | 14.46 |
| Basicity (pKb) | 1.51 |
| Magnetic susceptibility (χ) | -51.5·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.473 |
| Viscosity | 60.5 mPa·s (25 °C) |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 346.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -553.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2035 kJ mol⁻¹ |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P280, P301+P312, P305+P351+P338, P310 |
| NFPA 704 (fire diamond) | 1,2,4-Butanetriol: "1-2-0 |
| Flash point | 165°C |
| Autoignition temperature | 430 °C |
| Explosive limits | Explosive limits: 1.2–7.8% |
| Lethal dose or concentration | LD50 (oral, rat): 6400 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 5050 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 1 mg/m³ |
| Related compounds | |
| Related compounds |
1,2,3-Butanetriol
Glycerol Erythritol 1,4-Butanediol Ethylene glycol |